

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93422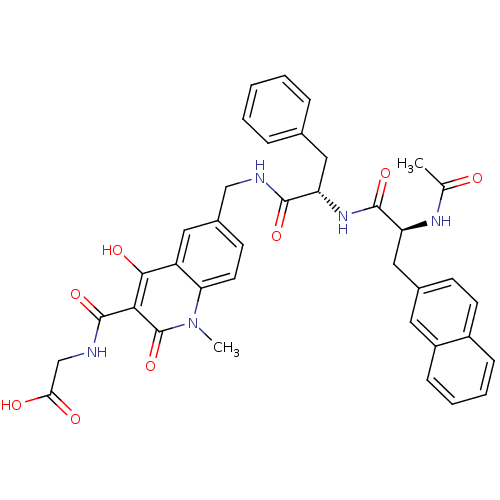 (PHD Inhibitor, 12{1,1,2}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93422 (PHD Inhibitor, 12{1,1,2}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93425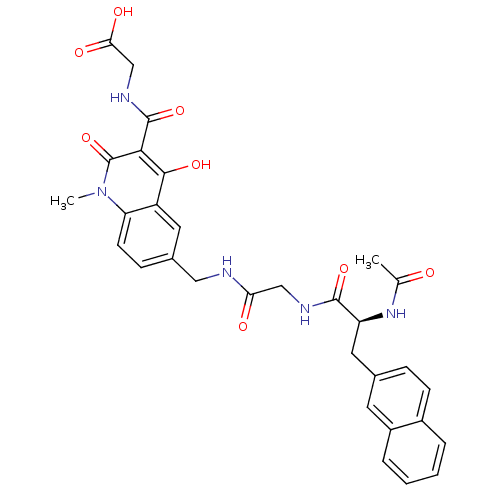 (PHD Inhibitor, 12{4,1,2}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93419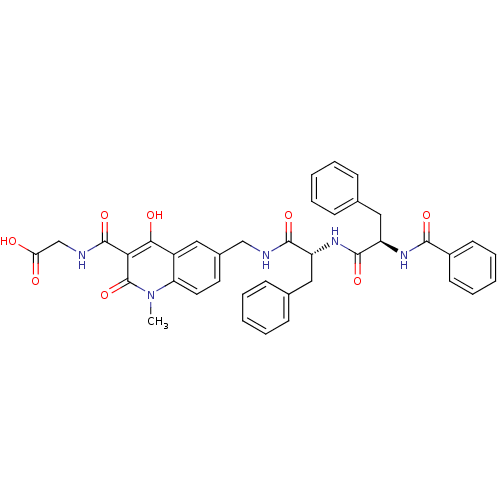 (PHD Inhibitor, 12{2,4,1}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93430 (PHD Inhibitor, 12{2,1,2}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93423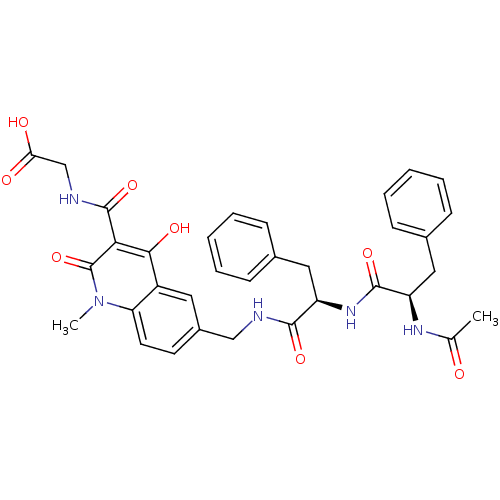 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93426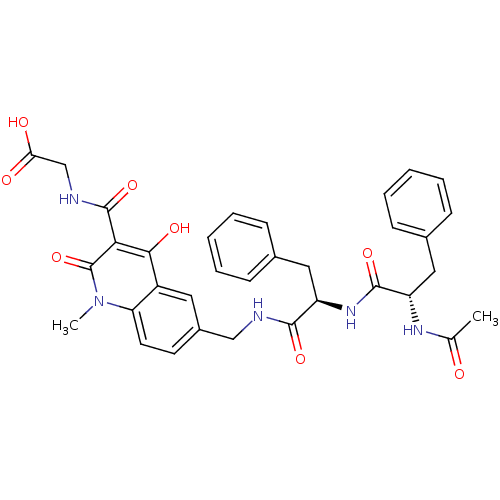 (PHD Inhibitor, 12{2,5,2}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93419 (PHD Inhibitor, 12{2,4,1}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93426 (PHD Inhibitor, 12{2,5,2}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93427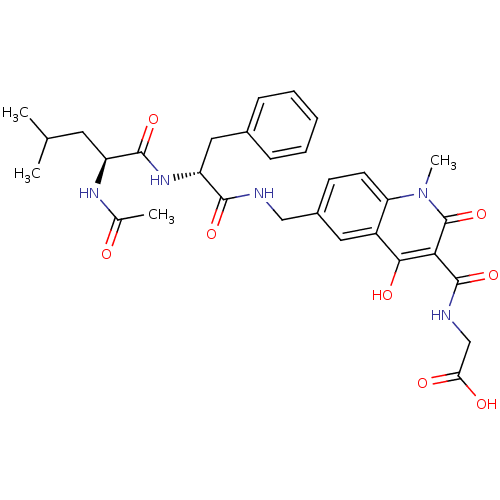 (PHD Inhibitor, 12{2,6,2}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93423 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93423 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93430 (PHD Inhibitor, 12{2,1,2}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93425 (PHD Inhibitor, 12{4,1,2}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93423 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93432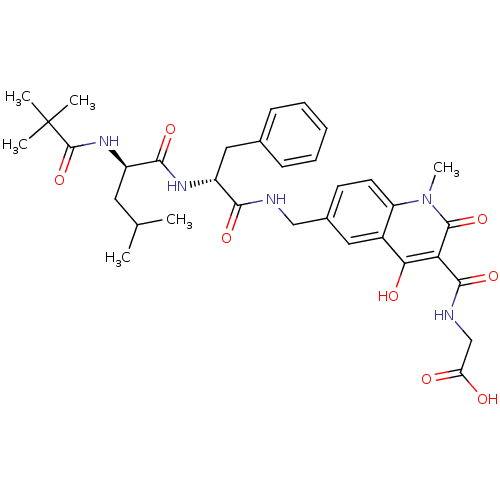 (PHD Inhibitor, 12{2,6,4}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93427 (PHD Inhibitor, 12{2,6,2}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93432 (PHD Inhibitor, 12{2,6,4}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93433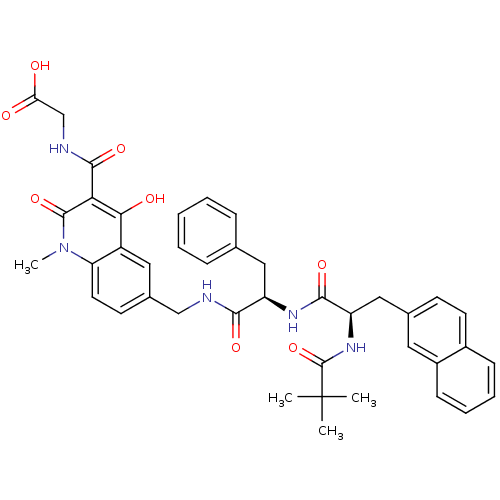 (PHD Inhibitor, 12{2,1,4}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93419 (PHD Inhibitor, 12{2,4,1}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93419 (PHD Inhibitor, 12{2,4,1}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93420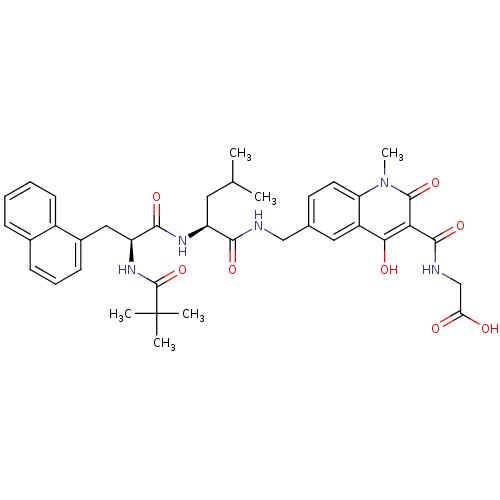 (PHD Inhibitor, 12{3,1,4}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93422 (PHD Inhibitor, 12{1,1,2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93423 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93419 (PHD Inhibitor, 12{2,4,1}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93418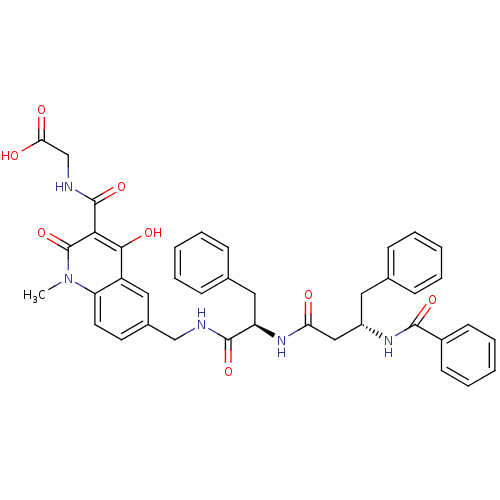 (PHD Inhibitor, 12{2,3,1}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93425 (PHD Inhibitor, 12{4,1,2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93426 (PHD Inhibitor, 12{2,5,2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93418 (PHD Inhibitor, 12{2,3,1}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93433 (PHD Inhibitor, 12{2,1,4}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93427 (PHD Inhibitor, 12{2,6,2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93420 (PHD Inhibitor, 12{3,1,4}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93423 (PHD Inhibitor, 12{2,3,2} | PHD Inhibitor, 12{2,4,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93430 (PHD Inhibitor, 12{2,1,2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM93421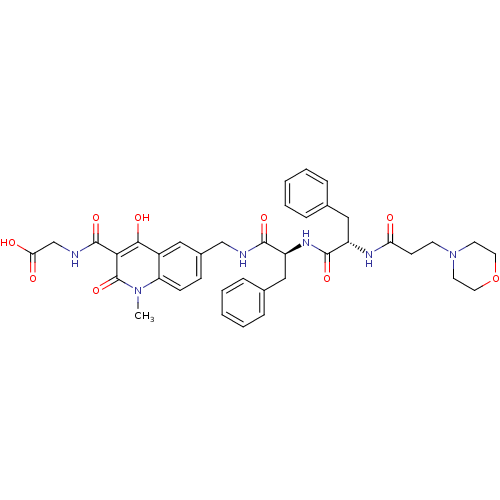 (PHD Inhibitor, 12{1,5,3}) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93428 (PHD Inhibitor, 12{2,3,4} | PHD Inhibitor, 12{2,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93412 (PHD Inhibitor, 8{2}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93432 (PHD Inhibitor, 12{2,6,4}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93416 (PHD Inhibitor, 8{6}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM93421 (PHD Inhibitor, 12{1,5,3}) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93415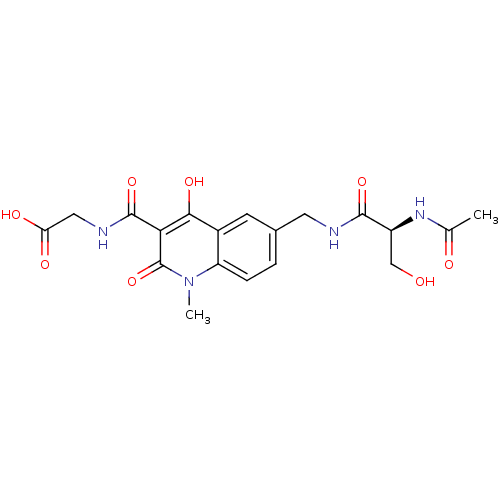 (PHD Inhibitor, 8{5}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93414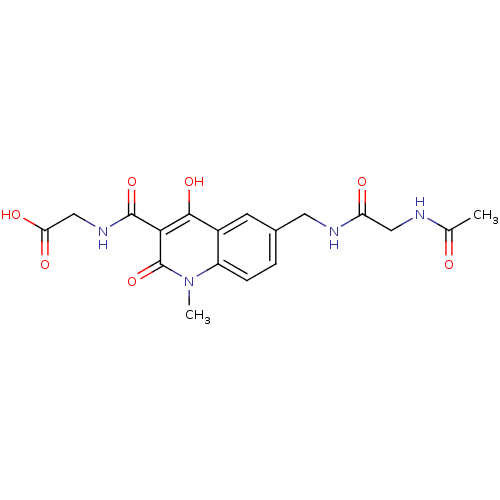 (PHD Inhibitor, 8{4}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93433 (PHD Inhibitor, 12{2,1,4}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93417 (PHD Inhibitor, 8{7}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 409 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM93411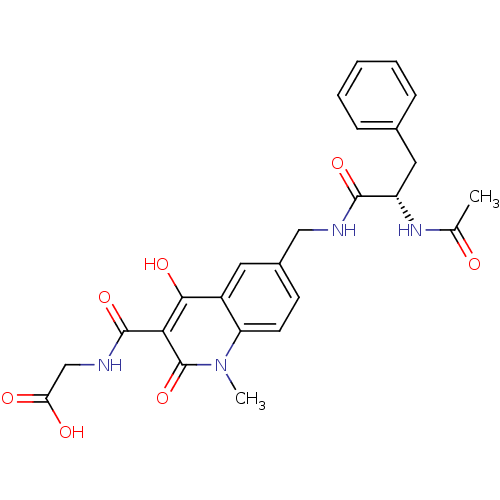 (PHD Inhibitor, 8{1}) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. | Assay Description PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI... | J Comb Chem 12: 676-86 (2010) Article DOI: 10.1021/cc100073a BindingDB Entry DOI: 10.7270/Q2B27SXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |