

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11551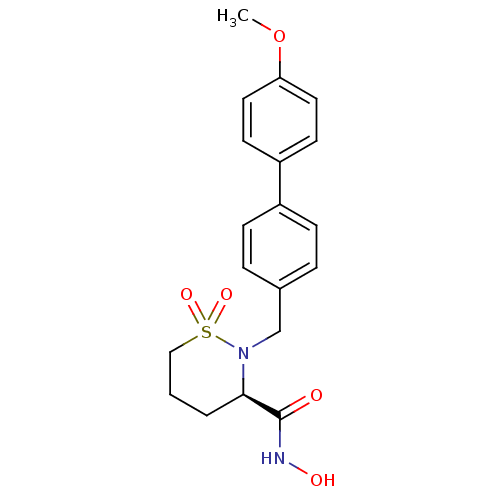 ((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11551 ((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11554 ((3R)-N-hydroxy-1,1-dioxo-2-{[4-(pyridin-4-yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11551 ((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11552 ((3R)-2-[(4-chloro-1,1-biphenyl-4-yl)methyl]-N-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11554 ((3R)-N-hydroxy-1,1-dioxo-2-{[4-(pyridin-4-yl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11553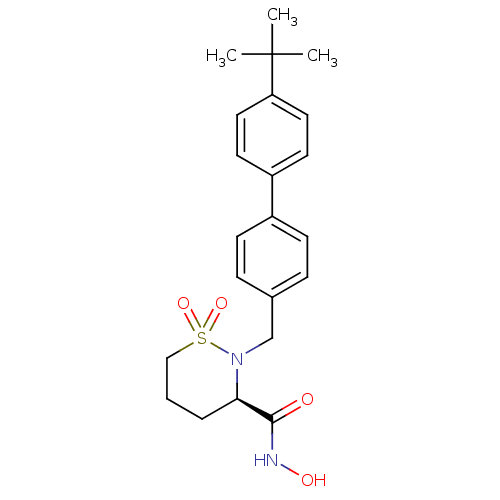 ((3R)-2-[(4-tert-butyl-1,1-biphenyl-4-yl)methyl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11552 ((3R)-2-[(4-chloro-1,1-biphenyl-4-yl)methyl]-N-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11548 (CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11553 ((3R)-2-[(4-tert-butyl-1,1-biphenyl-4-yl)methyl]-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11552 ((3R)-2-[(4-chloro-1,1-biphenyl-4-yl)methyl]-N-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11553 ((3R)-2-[(4-tert-butyl-1,1-biphenyl-4-yl)methyl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11554 ((3R)-N-hydroxy-1,1-dioxo-2-{[4-(pyridin-4-yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11546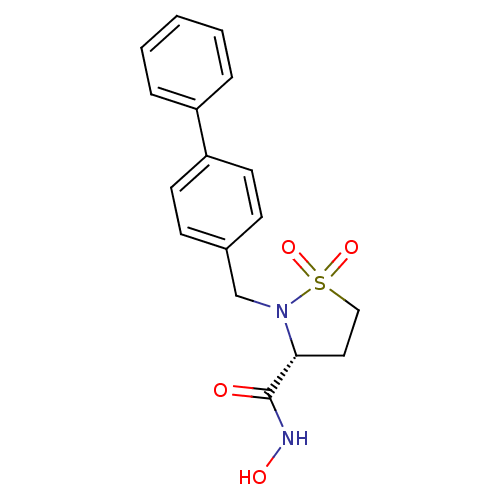 ((3R)-2-(1,1-biphenyl-4-ylmethyl)-N-hydroxyisothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11548 (CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11548 (CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11546 ((3R)-2-(1,1-biphenyl-4-ylmethyl)-N-hydroxyisothiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11552 ((3R)-2-[(4-chloro-1,1-biphenyl-4-yl)methyl]-N-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 318 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11547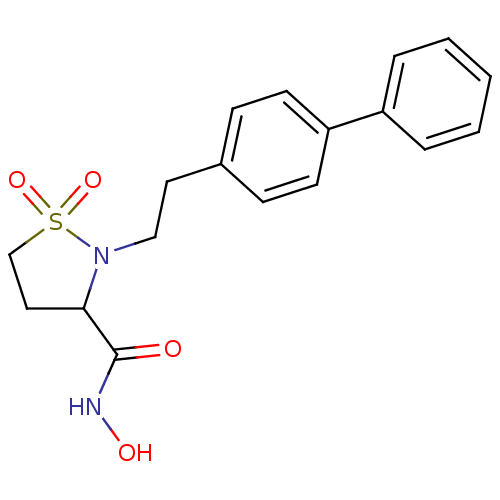 (2-[2-(1,1-biphenyl-4-yl)ethyl]-N-hydroxyisothiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11546 ((3R)-2-(1,1-biphenyl-4-ylmethyl)-N-hydroxyisothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11545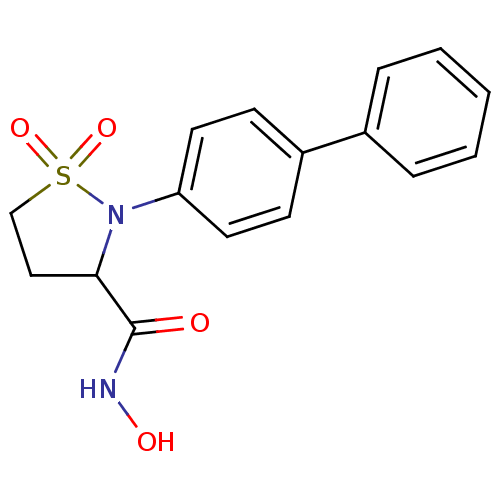 (2-(1,1-biphenyl-4-yl)-N-hydroxyisothiazolidine-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 667 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11547 (2-[2-(1,1-biphenyl-4-yl)ethyl]-N-hydroxyisothiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 702 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11545 (2-(1,1-biphenyl-4-yl)-N-hydroxyisothiazolidine-3-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11551 ((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 938 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11547 (2-[2-(1,1-biphenyl-4-yl)ethyl]-N-hydroxyisothiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11554 ((3R)-N-hydroxy-1,1-dioxo-2-{[4-(pyridin-4-yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.09E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11550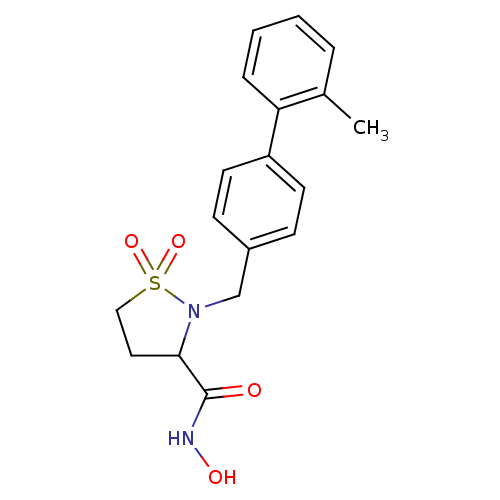 (N-hydroxy-2-[(2-methyl-1,1-biphenyl-4-yl)methyl]is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11549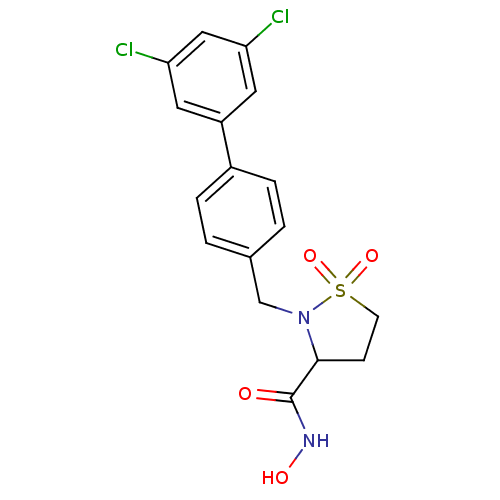 (2-[(3,5-dichloro-1,1-biphenyl-4-yl)methyl]-N-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11545 (2-(1,1-biphenyl-4-yl)-N-hydroxyisothiazolidine-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11553 ((3R)-2-[(4-tert-butyl-1,1-biphenyl-4-yl)methyl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.17E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11549 (2-[(3,5-dichloro-1,1-biphenyl-4-yl)methyl]-N-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11550 (N-hydroxy-2-[(2-methyl-1,1-biphenyl-4-yl)methyl]is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11550 (N-hydroxy-2-[(2-methyl-1,1-biphenyl-4-yl)methyl]is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11549 (2-[(3,5-dichloro-1,1-biphenyl-4-yl)methyl]-N-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11545 (2-(1,1-biphenyl-4-yl)-N-hydroxyisothiazolidine-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11547 (2-[2-(1,1-biphenyl-4-yl)ethyl]-N-hydroxyisothiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11546 ((3R)-2-(1,1-biphenyl-4-ylmethyl)-N-hydroxyisothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11548 (CHEMBL100570 | N-hydroxy-2-[(4-methoxy-1,1-bipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11550 (N-hydroxy-2-[(2-methyl-1,1-biphenyl-4-yl)methyl]is...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11549 (2-[(3,5-dichloro-1,1-biphenyl-4-yl)methyl]-N-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... | J Med Chem 47: 2981-3 (2004) Article DOI: 10.1021/jm049833g BindingDB Entry DOI: 10.7270/Q29W0CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||