

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12396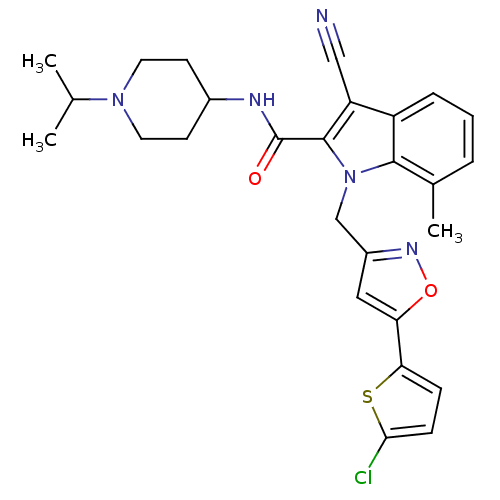 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12389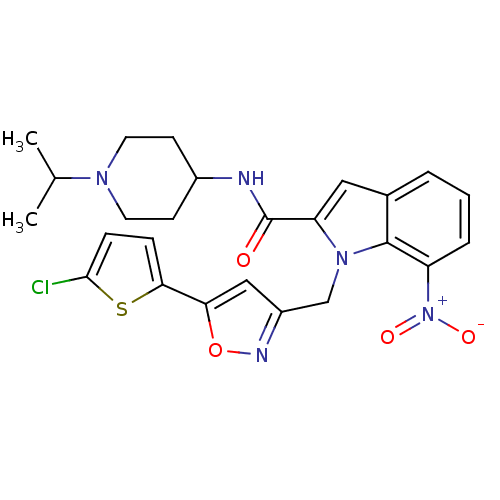 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12387 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12395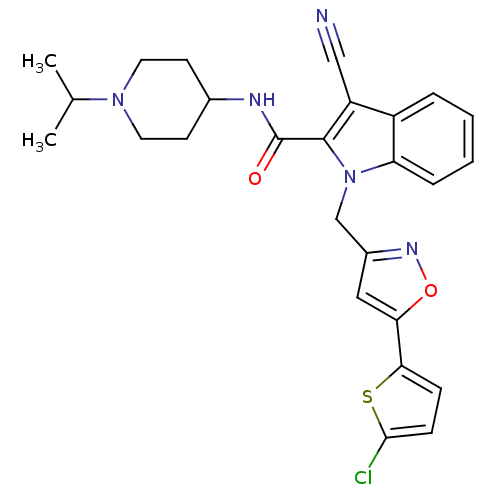 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12384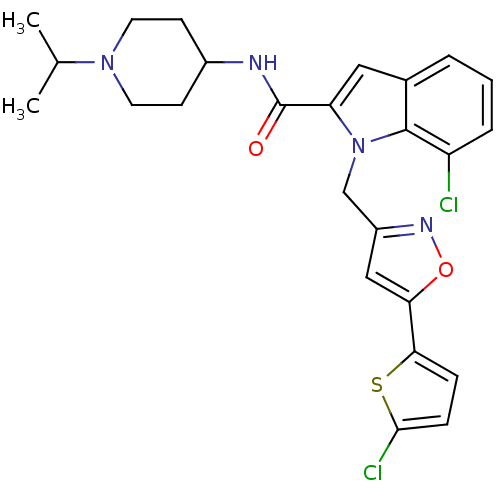 (2-Carboxyindole Scaffold 35 | 7-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12388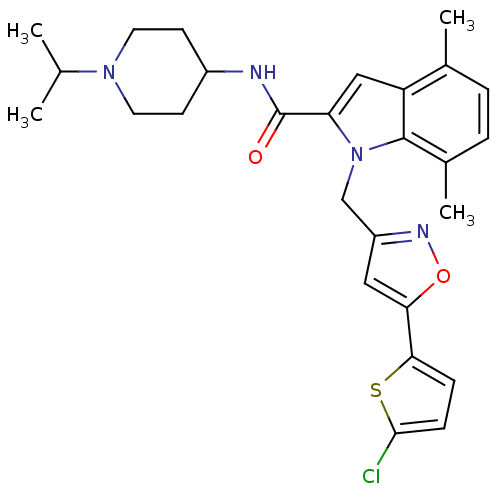 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12381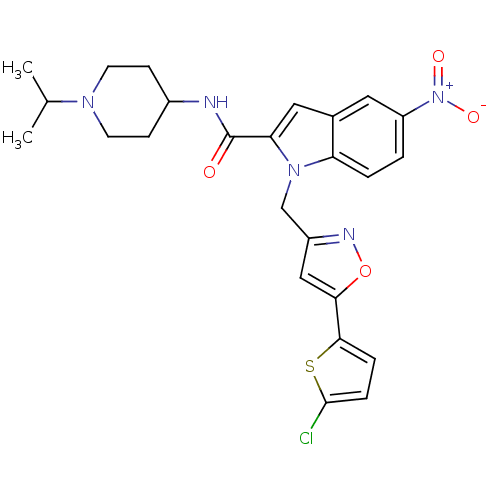 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12379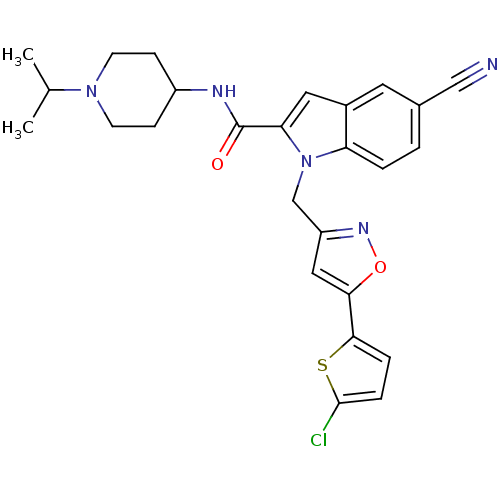 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12391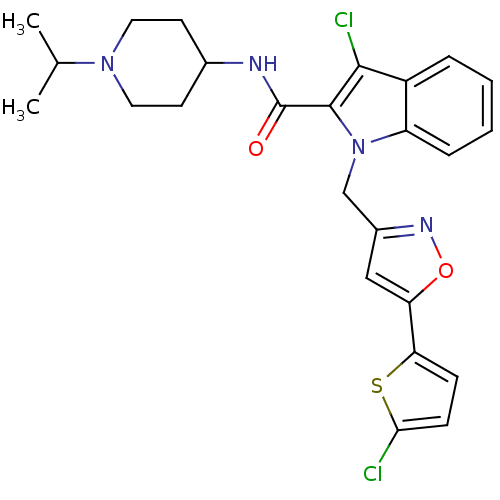 (2-Carboxyindole Scaffold 23 | 3-chloro-1-{[5-(5-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12392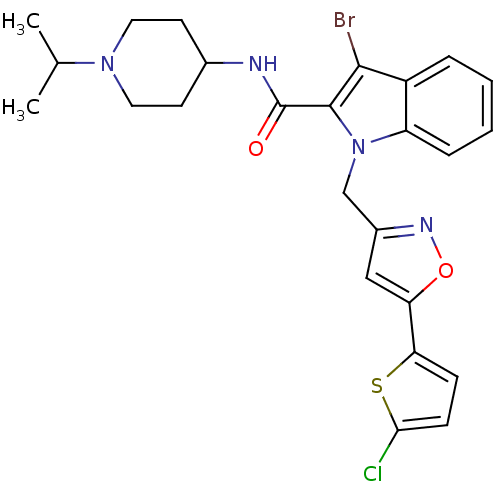 (2-Carboxyindole Scaffold 24 | 3-Bromo-1-[5-(5-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12390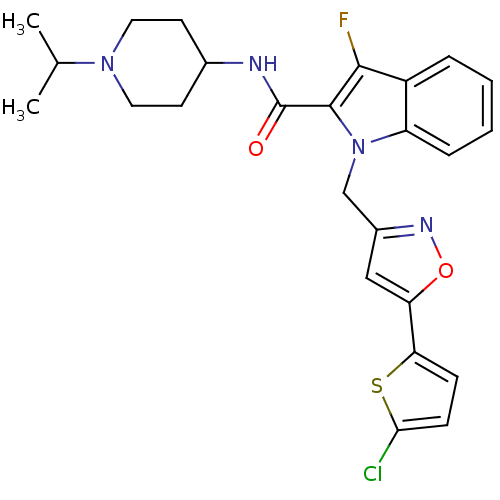 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12385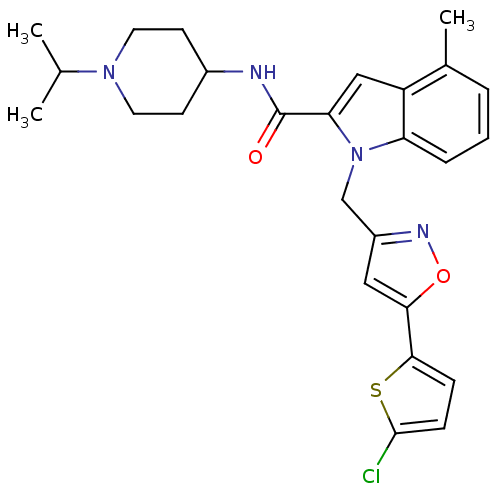 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372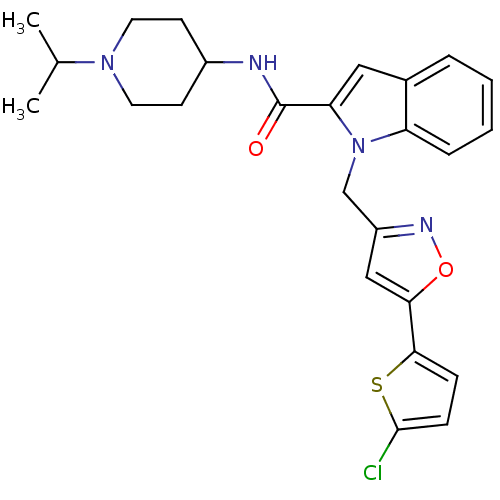 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12374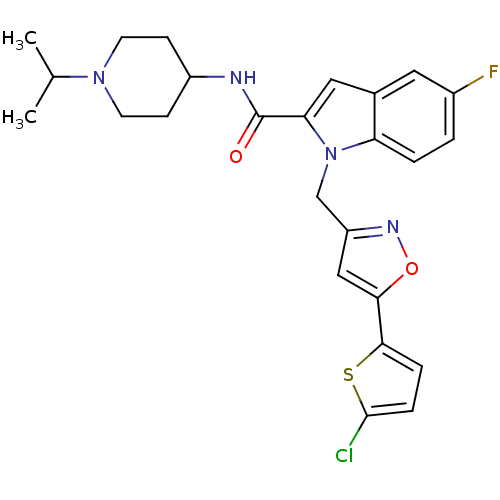 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12400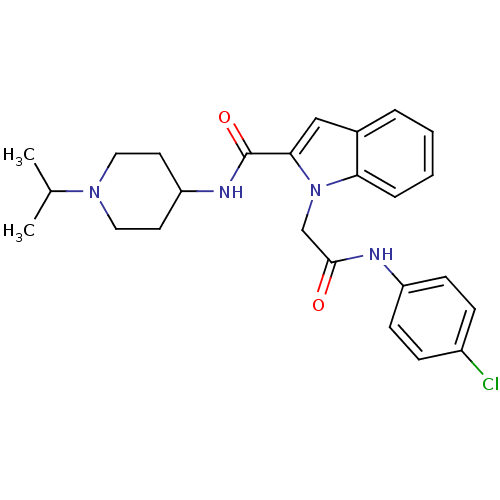 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12393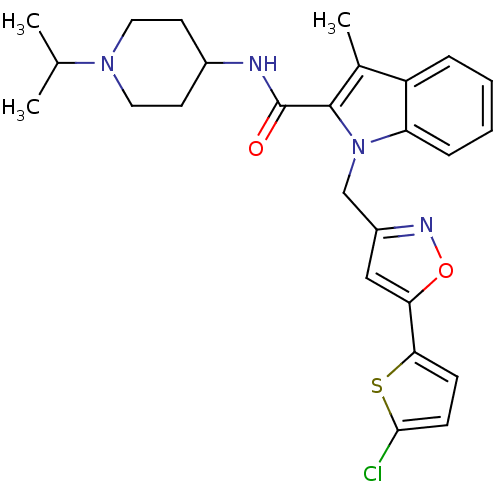 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12375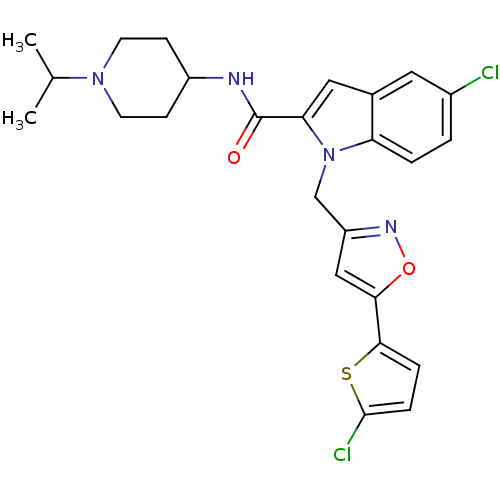 (2-Carboxyindole Scaffold 26 | 5-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12383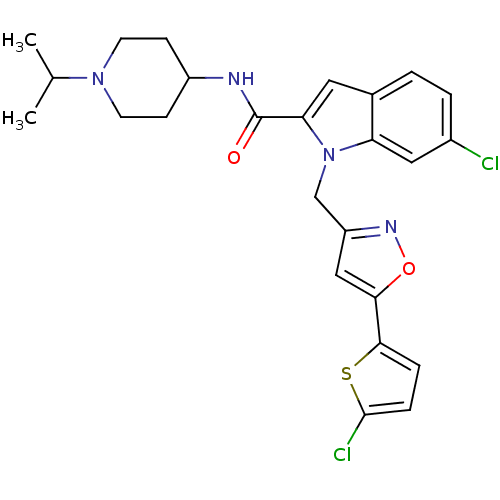 (2-Carboxyindole Scaffold 34 | 6-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12382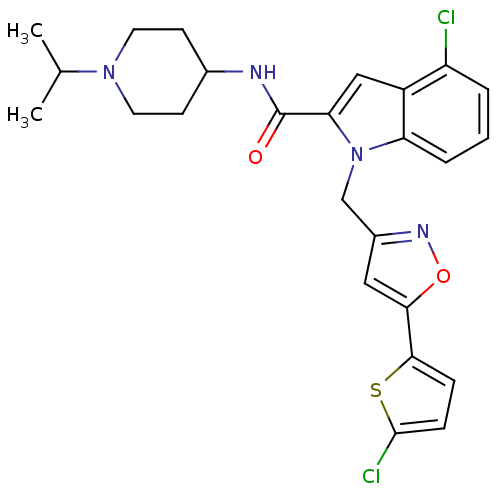 (2-Carboxyindole Scaffold 33 | 4-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12397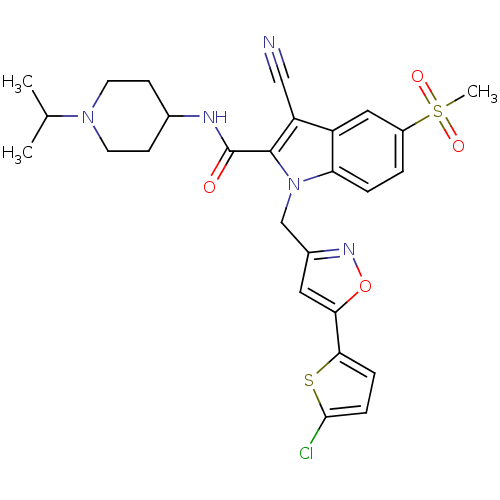 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12378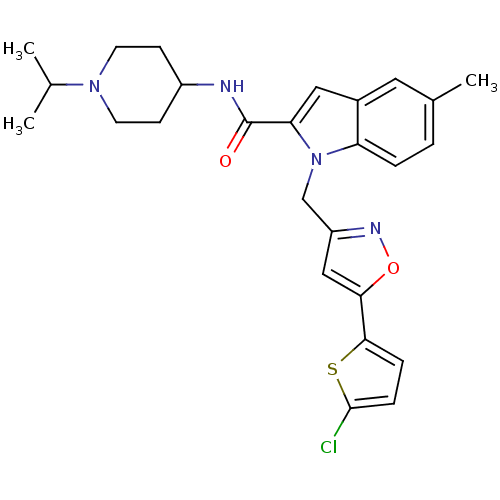 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12376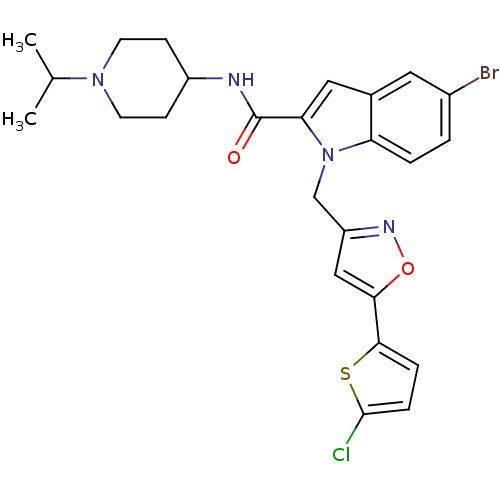 (2-Carboxyindole Scaffold 27 | 5-Bromo-1-[5-(5-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12386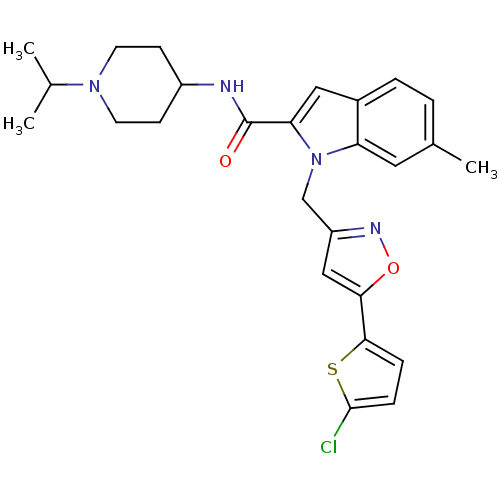 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12380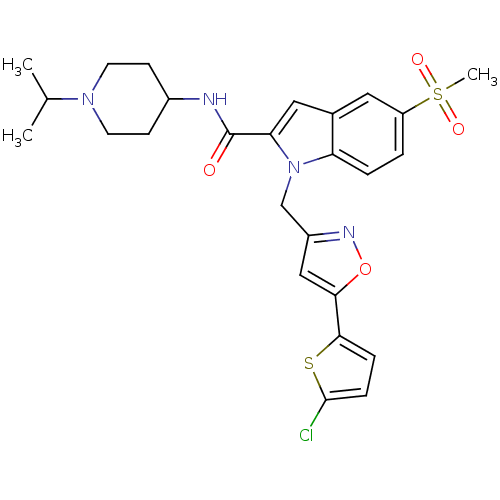 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12402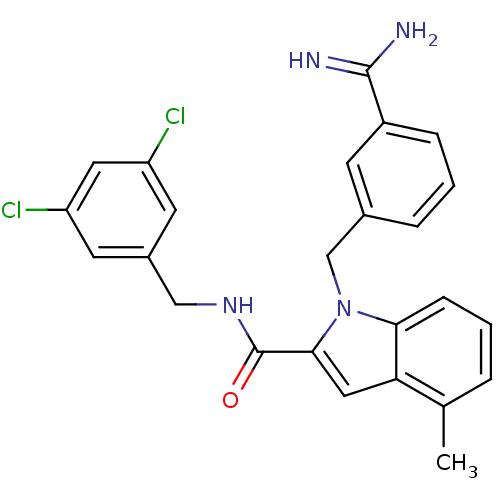 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12401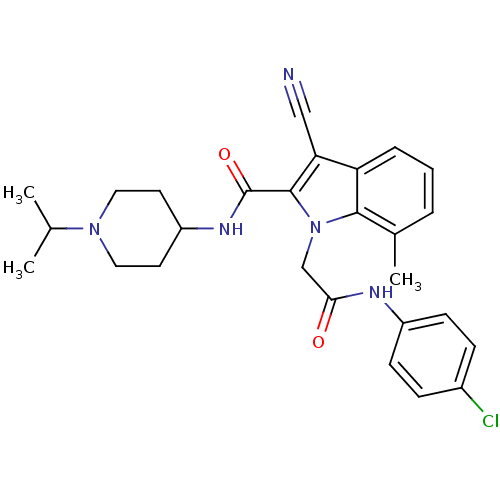 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-3-cyano-7-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12394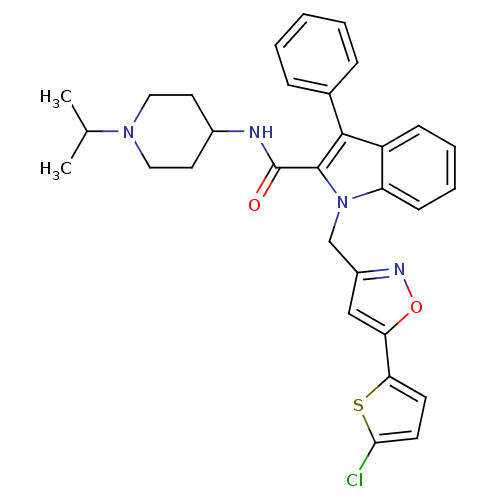 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12398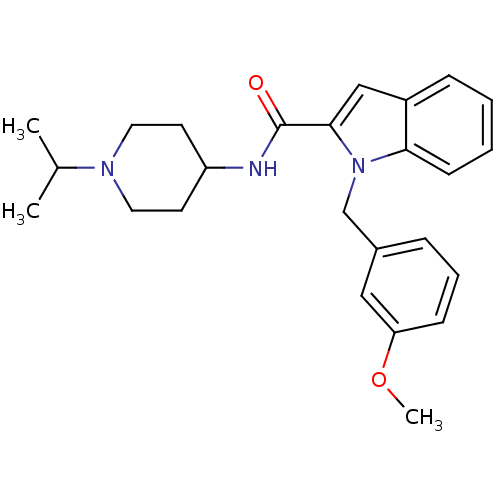 (1-(3-Methoxy-benzyl)-1H-indole-2-carboxylic acid (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12399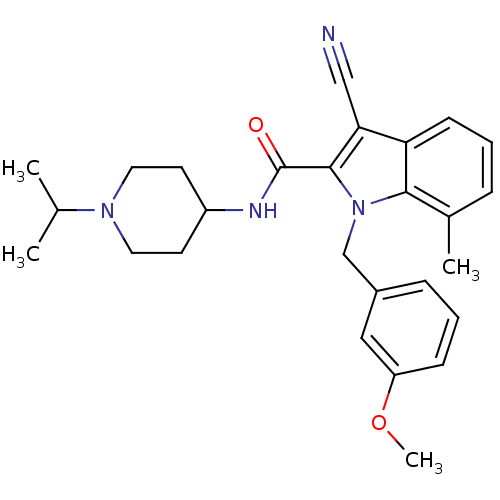 (2-Carboxyindole Scaffold 44 | 3-Cyano-1-(3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12377 (2-Carboxyindole Scaffold 28 | 5-(benzyloxy)-1-{[5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 835 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||