

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13662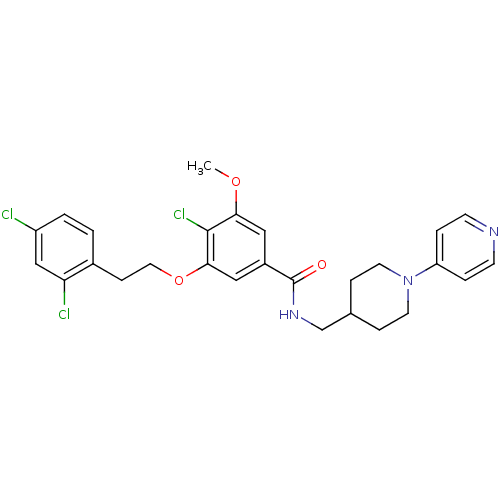 (3-Oxybenzamide 48 | 4-chloro-3-[2-(2,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13668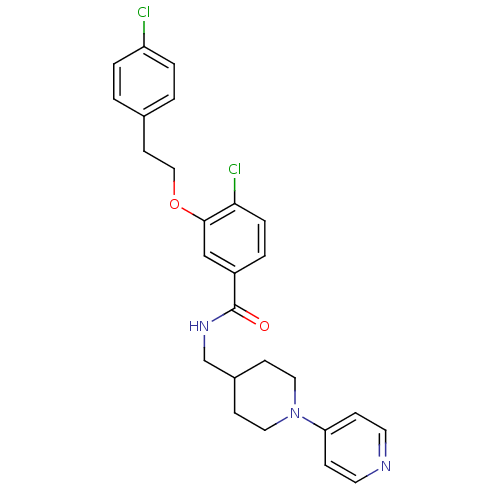 (3-Oxybenzamide 54 | 4-chloro-3-[2-(4-chlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13663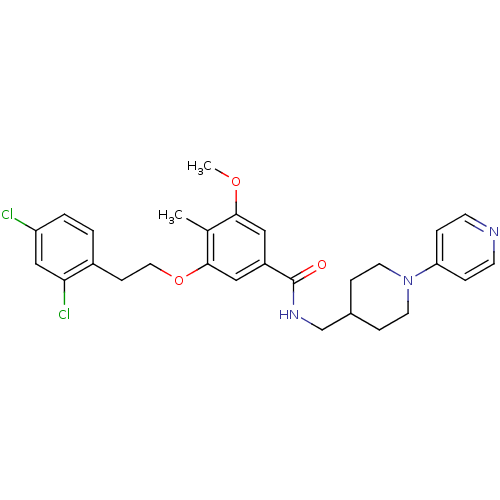 (3-Oxybenzamide 49 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13664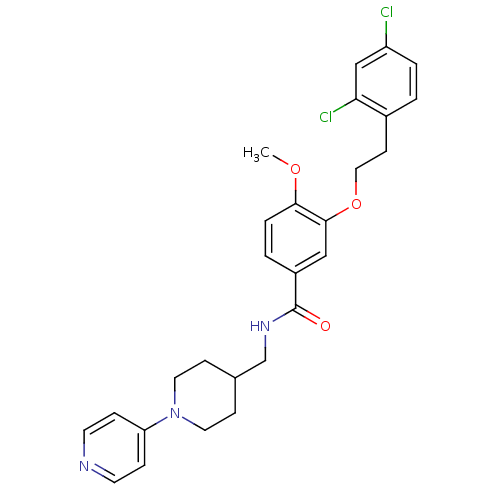 (3-Oxybenzamide 50 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13615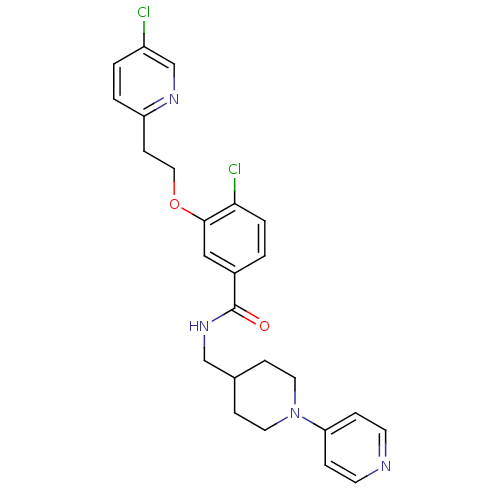 (3-Oxybenzamide 1 | 4-chloro-3-[2-(5-chloropyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13659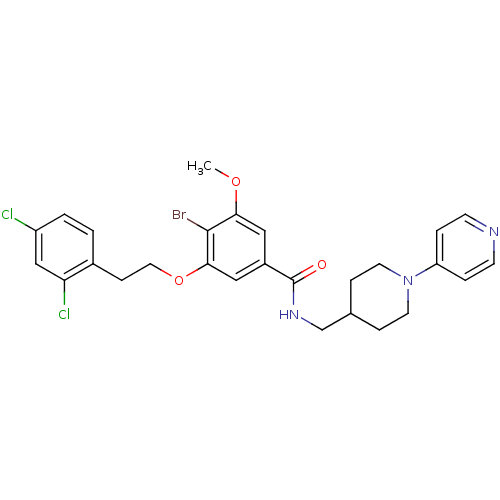 (3-Oxybenzamide 45 | 4-bromo-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13670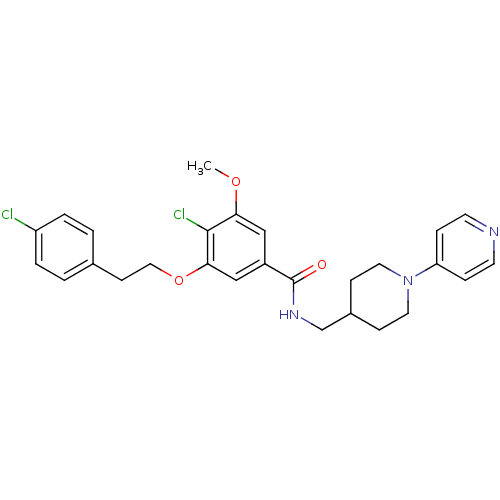 (3-Oxybenzamide 56 | 4-chloro-3-[2-(4-chlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13652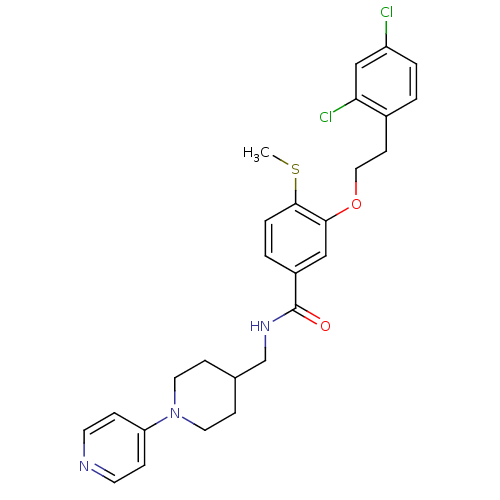 (3-Oxybenzamide 38 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13645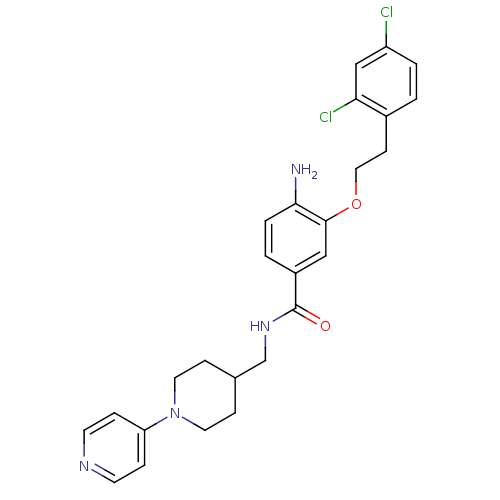 (3-Oxybenzamide 31 | 4-amino-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13619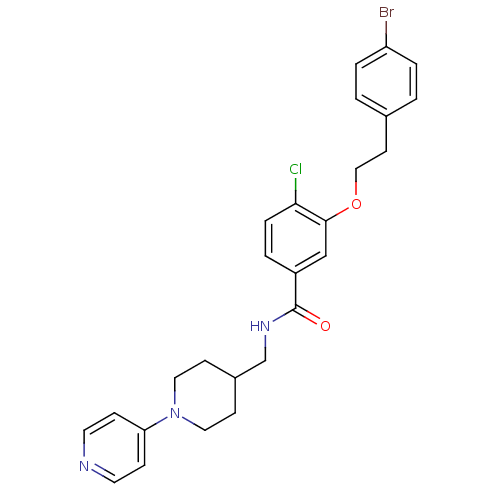 (3-Oxybenzamide 5 | 3-[2-(4-bromophenyl)ethoxy]-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13642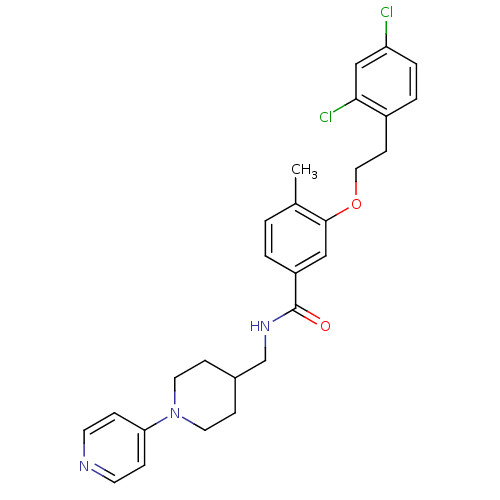 (3-Oxybenzamide 28 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13618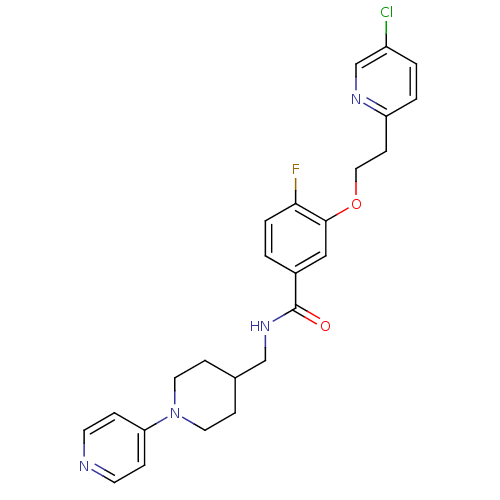 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13658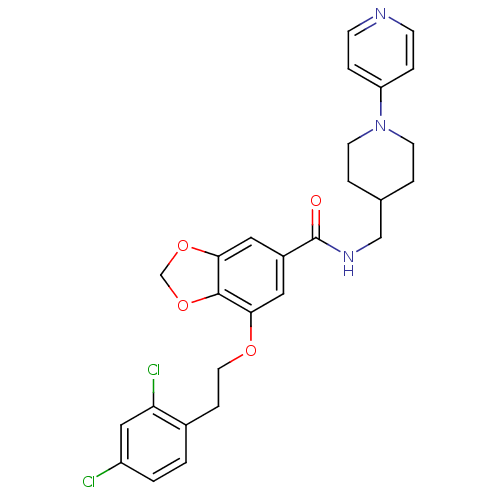 (3-Oxybenzamide 44 | 7-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13617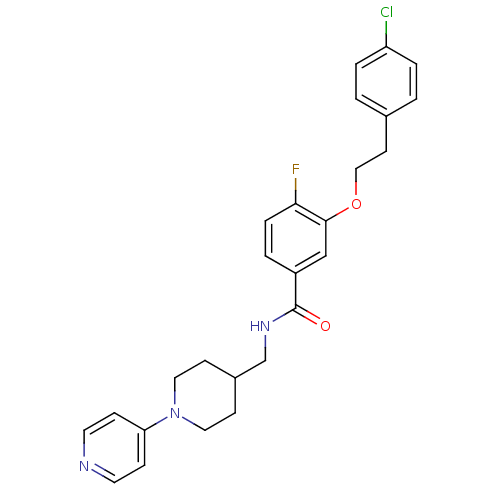 (3-Oxybenzamide 3 | 3-[2-(4-chlorophenyl)ethoxy]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13665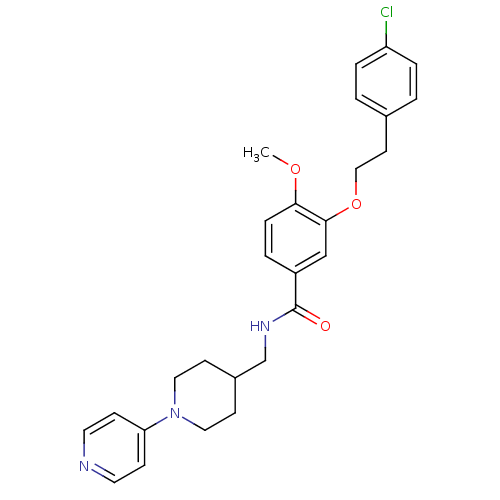 (3-Oxybenzamide 51 | 3-[2-(4-chlorophenyl)ethoxy]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13667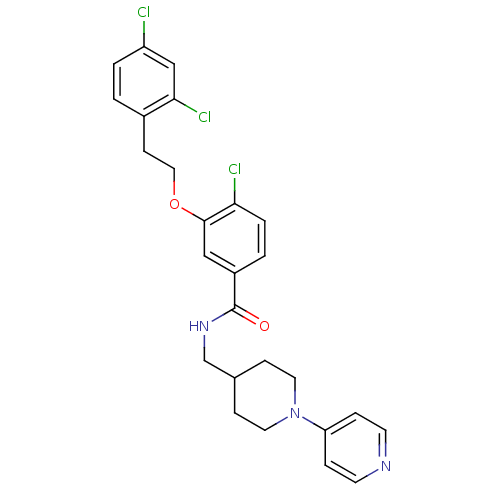 (3-Oxybenzamide 53 | 4-chloro-3-[2-(2,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13644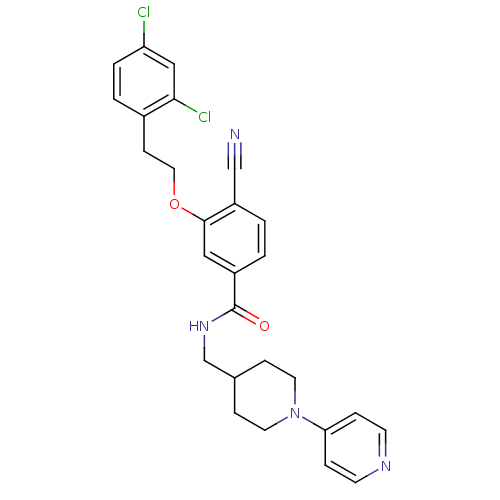 (3-Oxybenzamide 30 | 4-cyano-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13646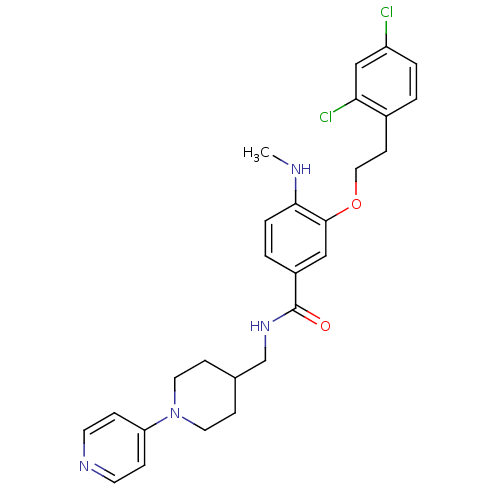 (3-Oxybenzamide 32 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13616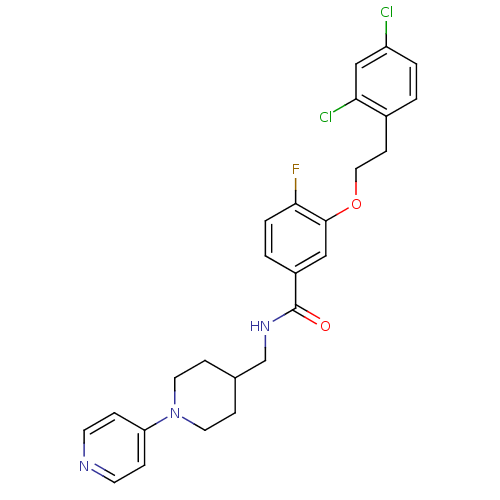 (3-Oxybenzamide 2 | 3-[2-(2,4-dichlorophenyl)ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13656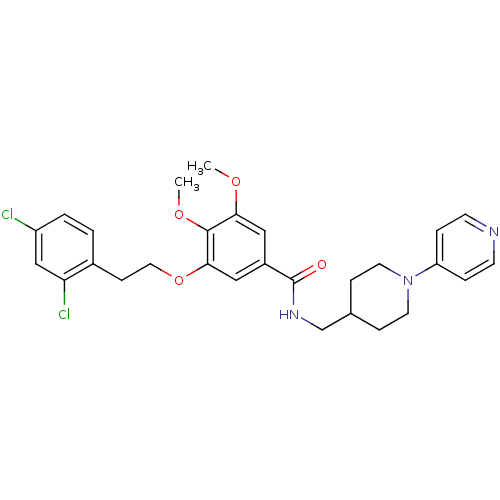 (3-Oxybenzamide 42 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13651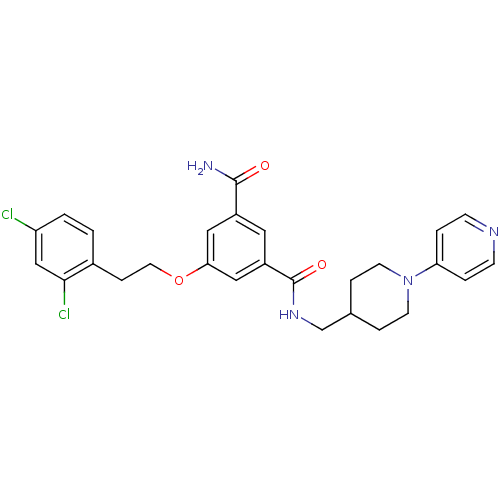 (3-Oxybenzamide 37 | 5-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13666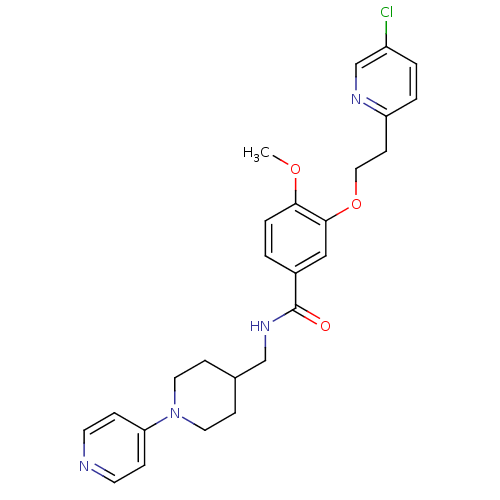 (3-Oxybenzamide 52 | 3-[2-(5-chloropyridin-2-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13673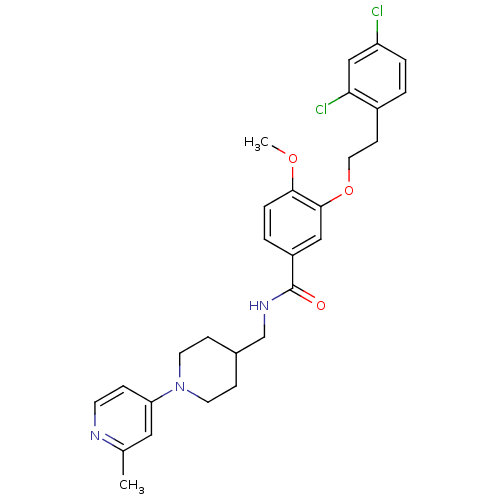 (3-Oxybenzamide 59 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13653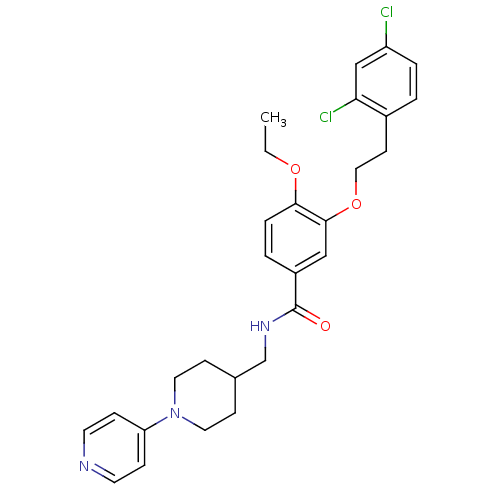 (3-Oxybenzamide 39 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13629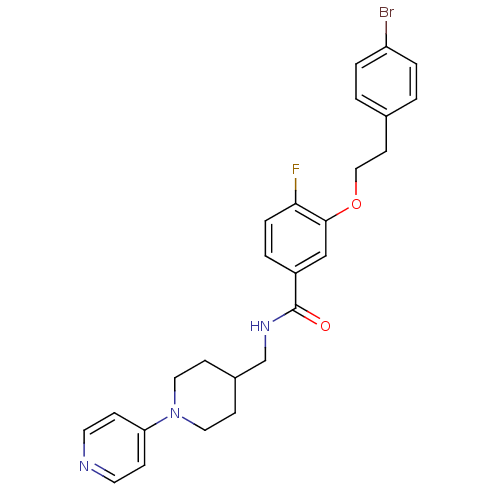 (3-Oxybenzamide 15 | 3-[2-(4-bromophenyl)ethoxy]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13647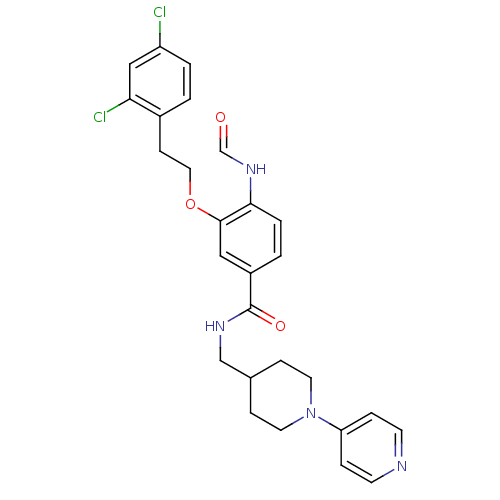 (3-Oxybenzamide 33 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13640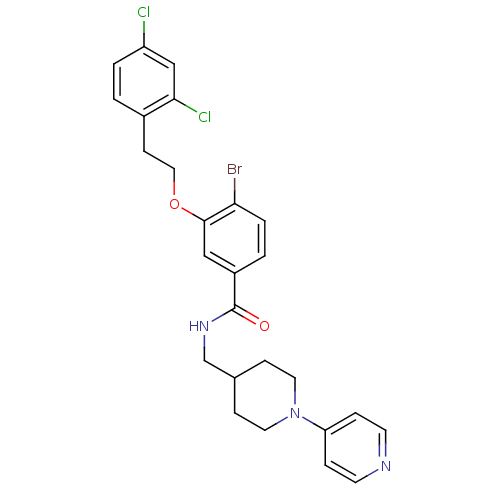 (3-Oxybenzamide 26 | 4-bromo-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13660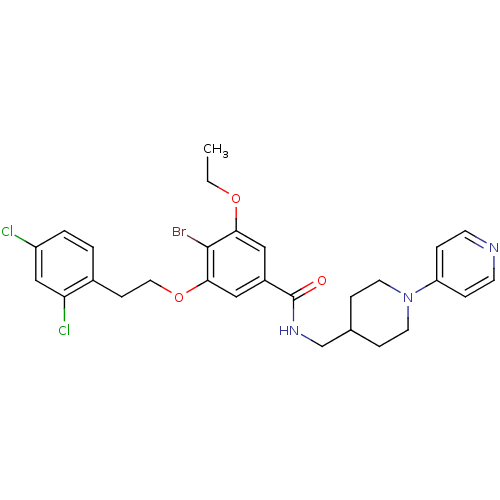 (3-Oxybenzamide 46 | 4-bromo-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13620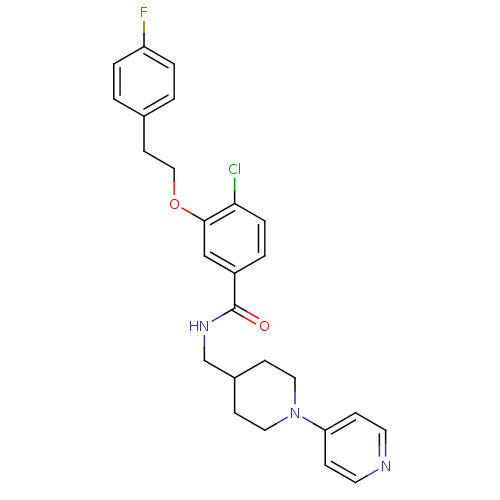 (3-Oxybenzamide 6 | 4-chloro-3-[2-(4-fluorophenyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13639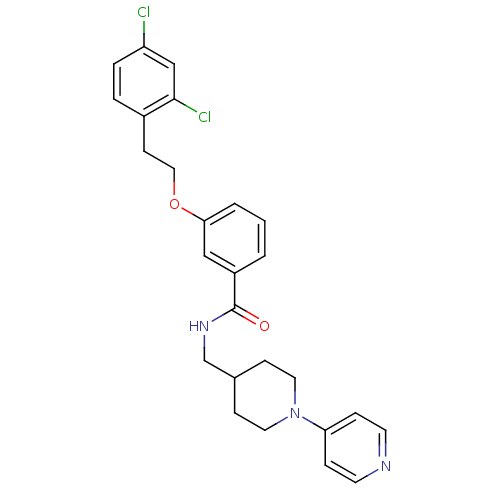 (3-Oxybenzamide 25 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13674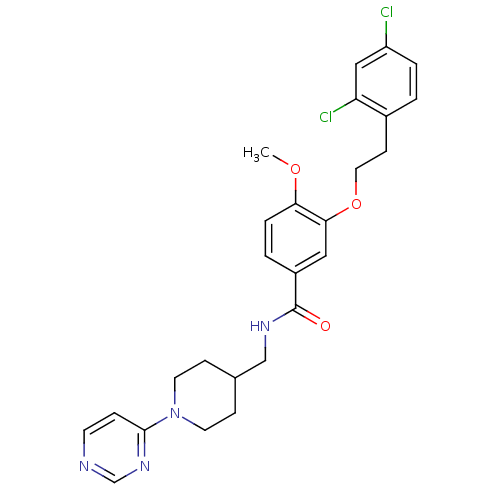 (3-Oxybenzamide 60 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13621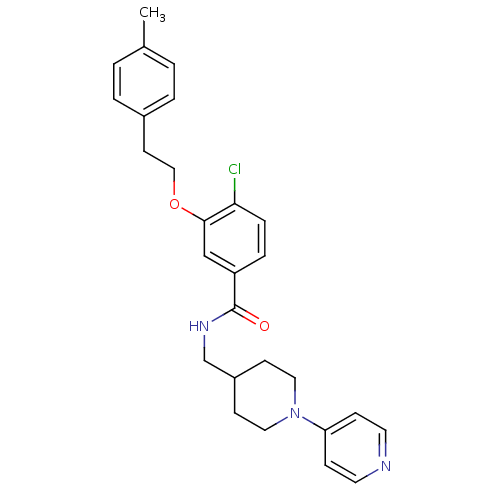 (3-Oxybenzamide 7 | 4-chloro-3-[2-(4-methylphenyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | -39.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13655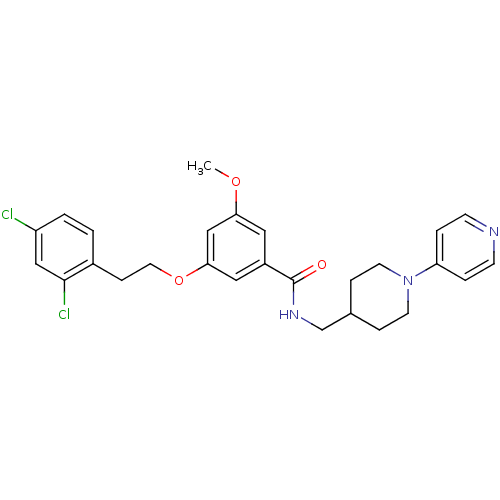 (3-Oxybenzamide 41 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13643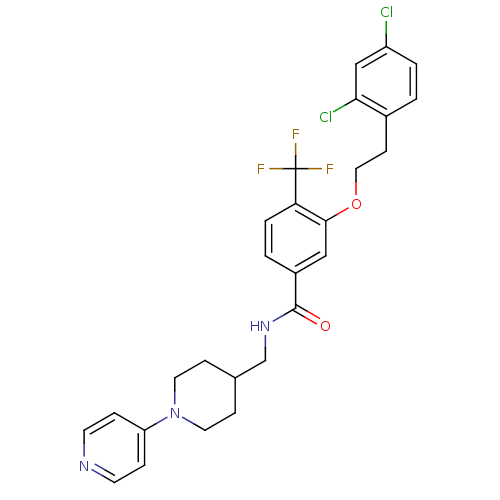 (3-Oxybenzamide 29 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13689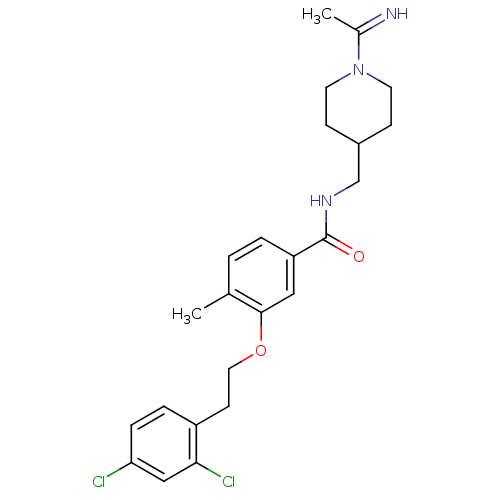 (3-Oxybenzamide 75 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13671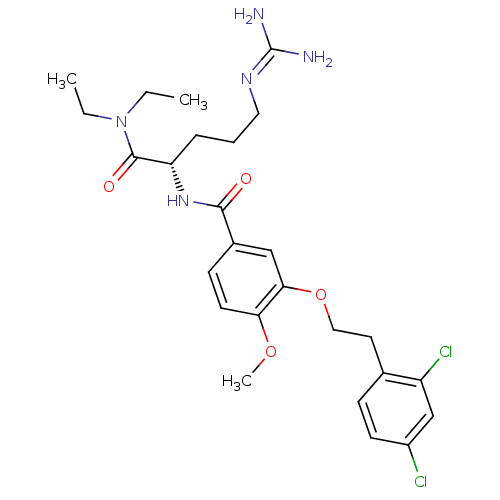 ((2S)-5-carbamimidamido-2-({3-[2-(2,4-dichloropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13641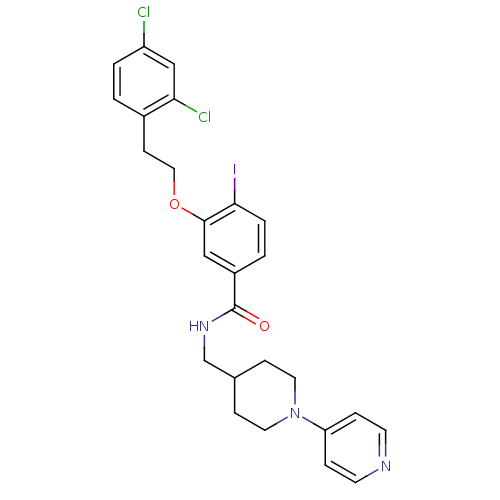 (3-Oxybenzamide 27 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13706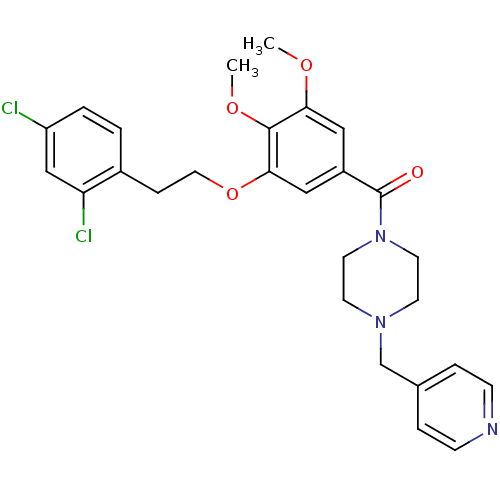 (1-({3-[2-(2,4-dichlorophenyl)ethoxy]-4,5-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13687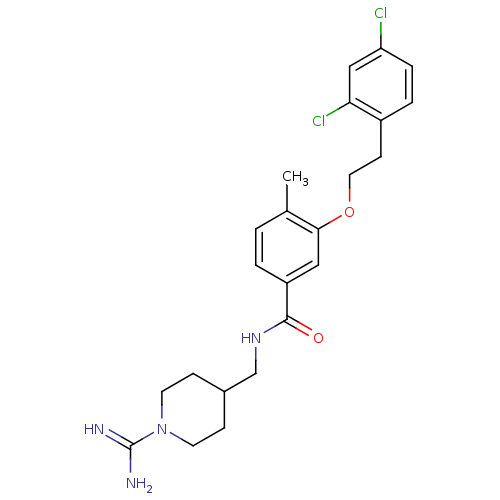 (3-Oxybenzamide 73 | N-[(1-carbamimidoylpiperidin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13672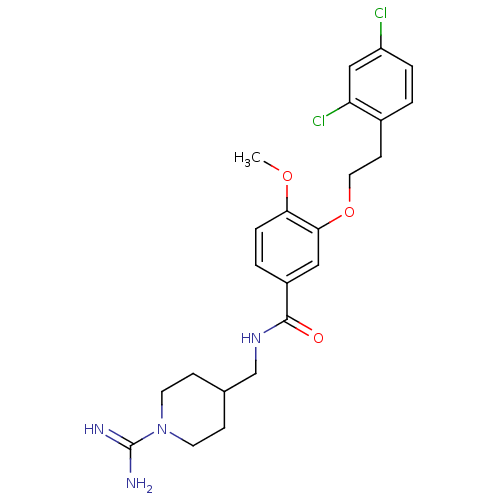 (3-Oxybenzamide 58 | N-[(1-carbamimidoylpiperidin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13630 (3-Oxybenzamide 16 | 4-fluoro-3-[2-(4-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13631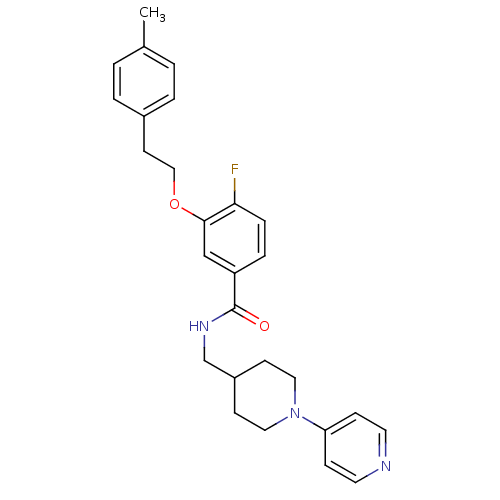 (3-Oxybenzamide 17 | 4-fluoro-3-[2-(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13654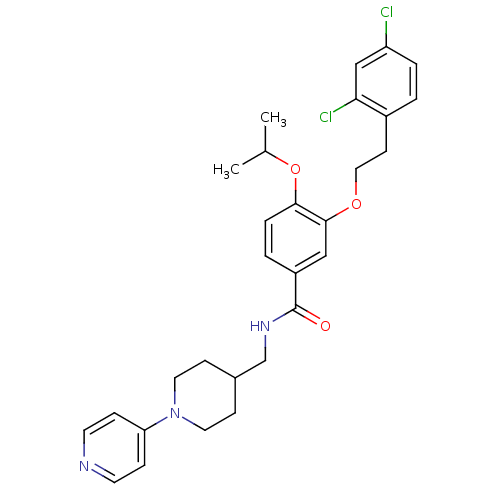 (3-Oxybenzamide 40 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13657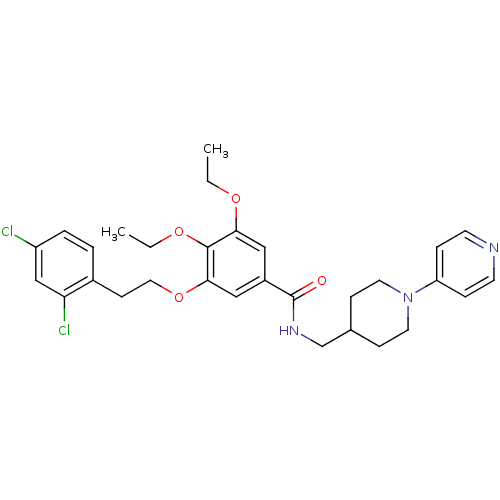 (3-Oxybenzamide 43 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13675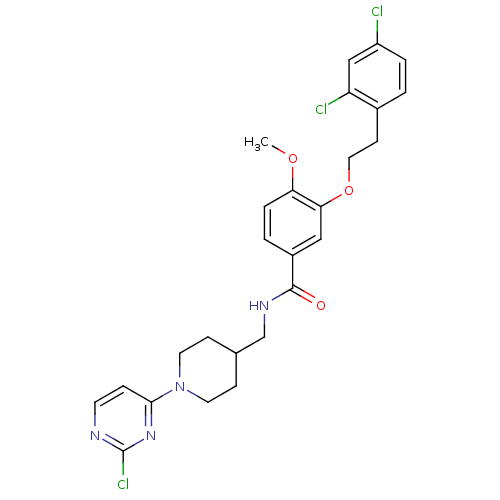 (3-Oxybenzamide 61 | N-{[1-(2-chloropyrimidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13669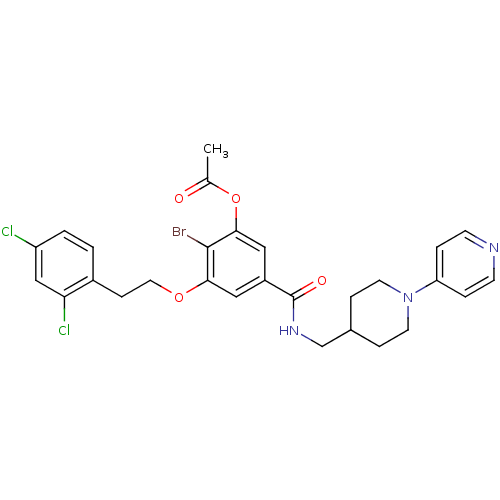 (2-bromo-3-[2-(2,4-dichlorophenyl)ethoxy]-5-({[1-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13707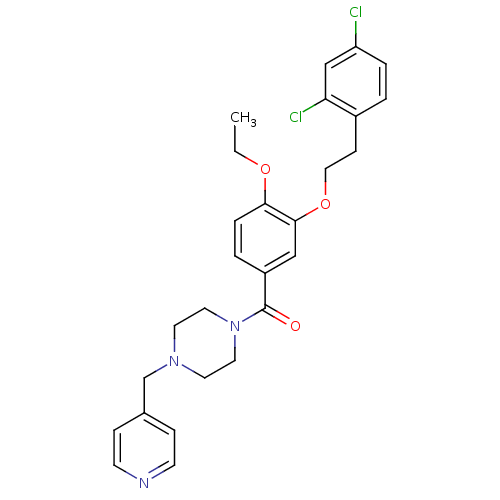 (1-({3-[2-(2,4-dichlorophenyl)ethoxy]-4-ethoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13632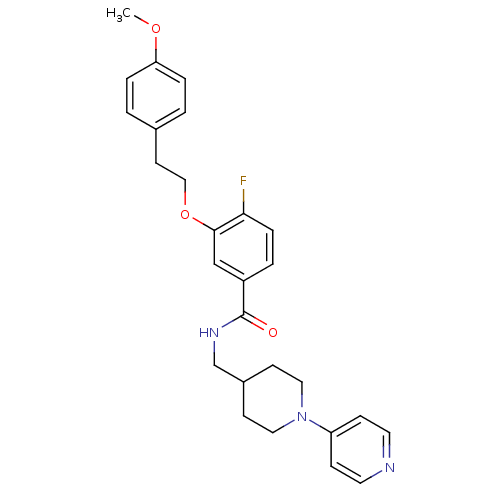 (3-Oxybenzamide 18 | 4-fluoro-3-[2-(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13622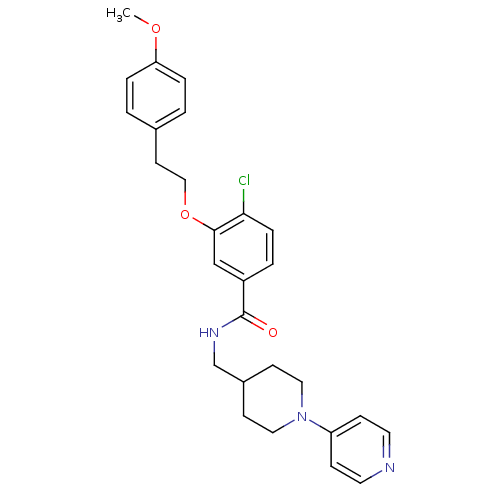 (3-Oxybenzamide 8 | 4-chloro-3-[2-(4-methoxyphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 314 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13648 (3-Oxybenzamide 34 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |