

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136527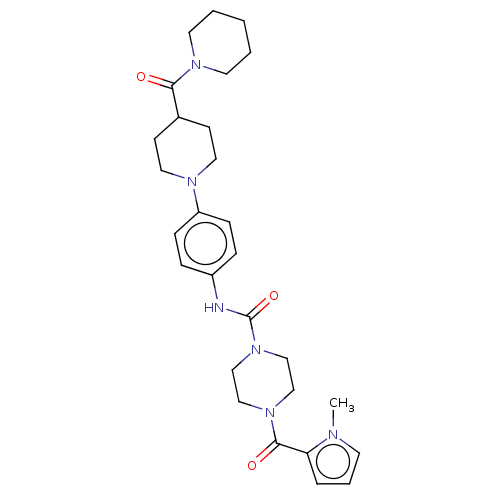 (US8865714, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136525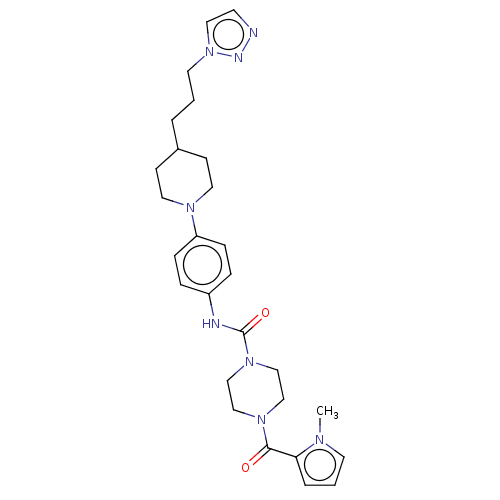 (US8865714, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136523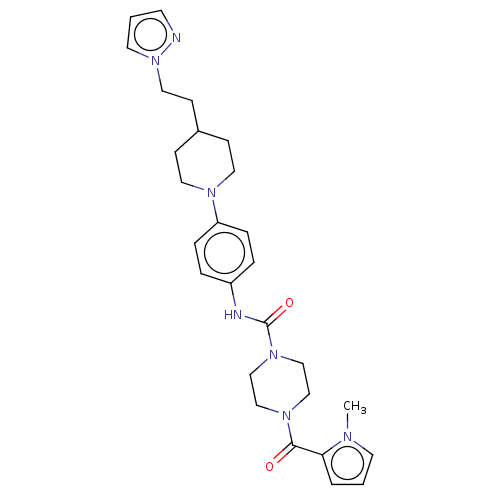 (US8865714, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136519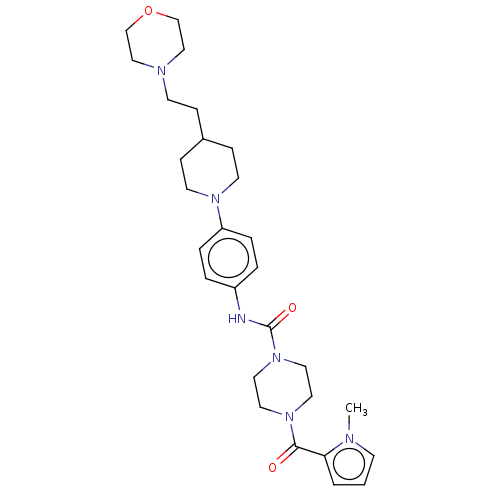 (US8865714, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136524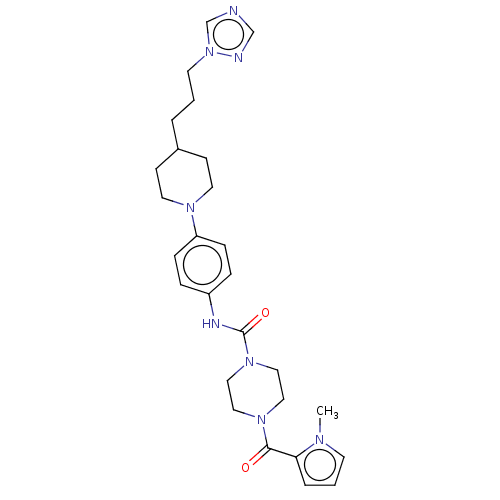 (US8865714, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136517 (US8865714, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136514 (US8865714, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136529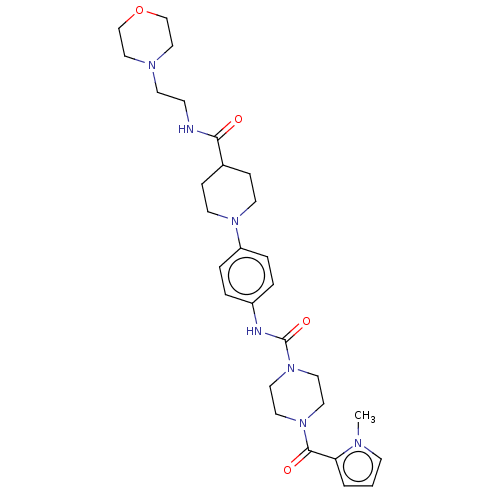 (US8865714, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136528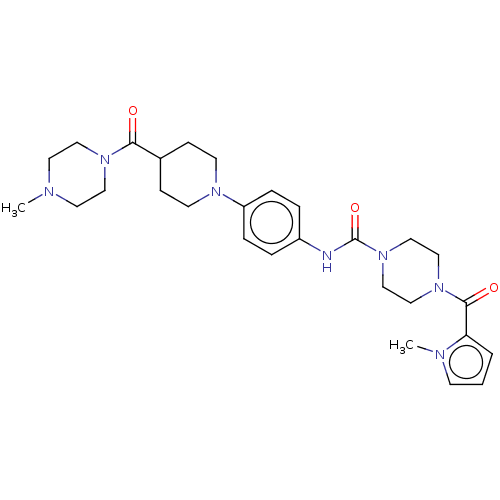 (US8865714, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136530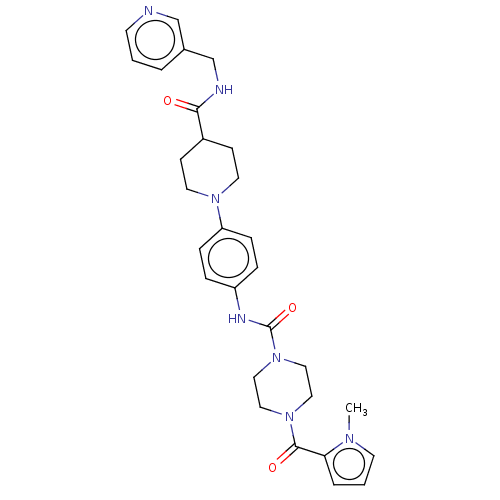 (US8865714, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136521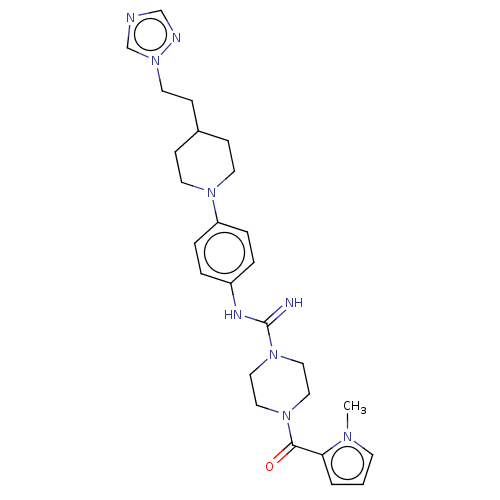 (US8865714, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136518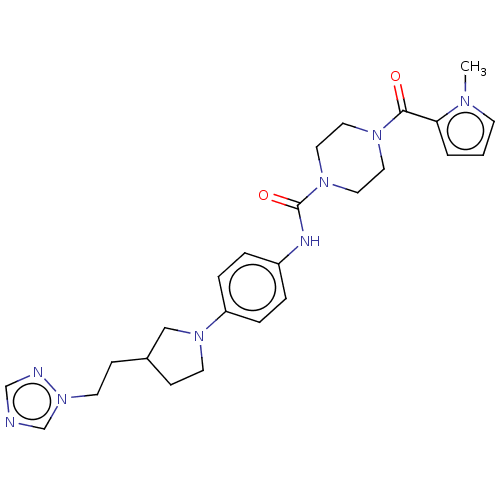 (US8865714, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136520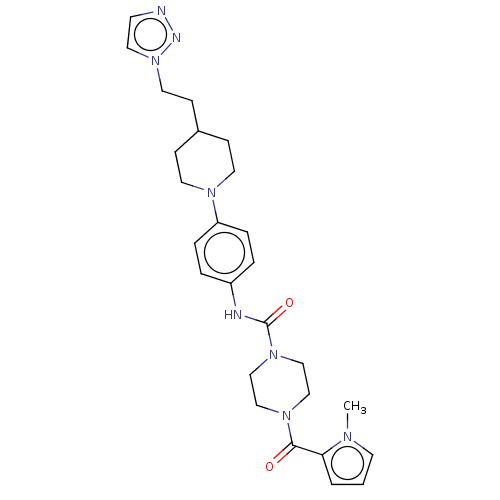 (US8865714, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136516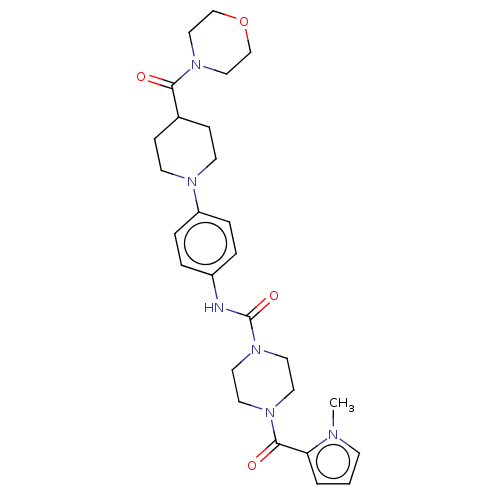 (US8865714, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136522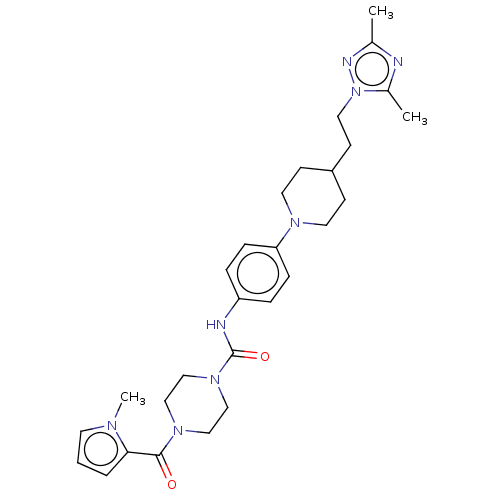 (US8865714, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136515 (US8865714, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136526 (US8865714, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM124939 (US8765750, Reference Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 106 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM124943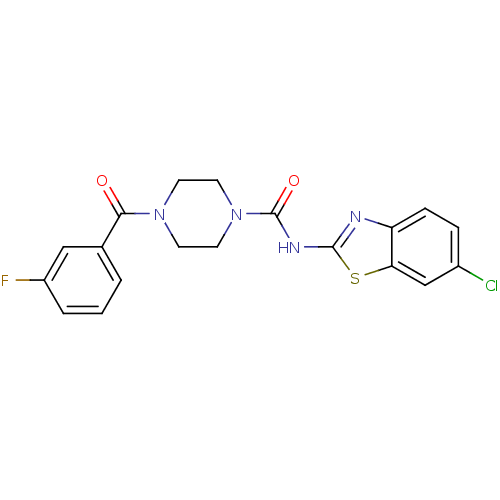 (US8765750, Reference Example 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 204 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM124940 (US8765750, Reference Example 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 222 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM124941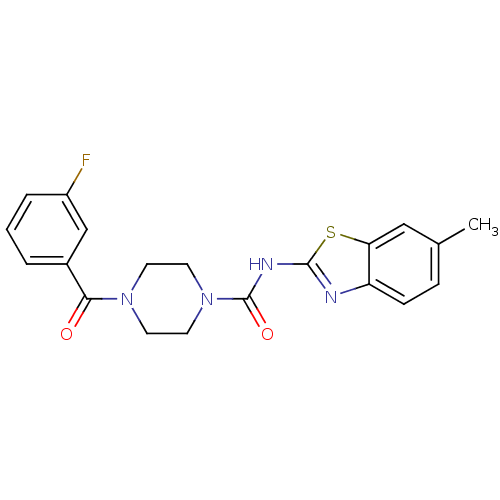 (US8765750, Reference Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM124942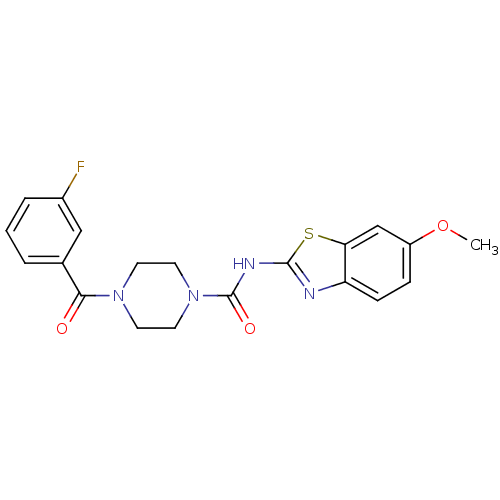 (US8765750, Reference Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 318 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136534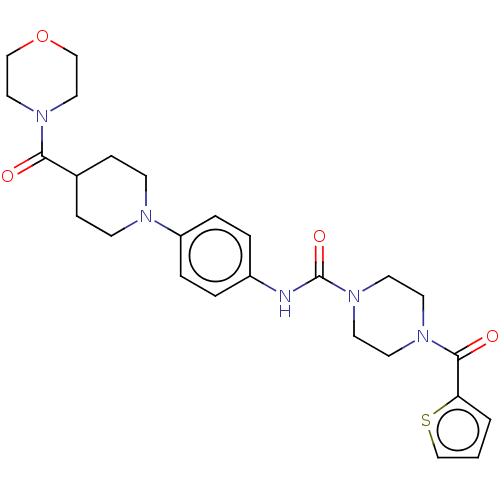 (US8865714, Reference Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136541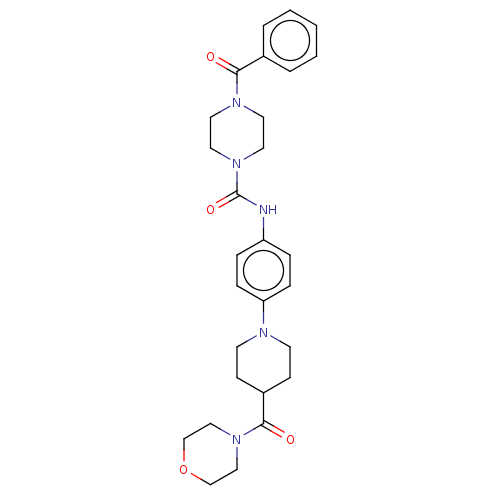 (US8865714, Reference Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 437 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136535 (US8865714, Reference Example 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 732 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136542 (US8865714, Reference Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136531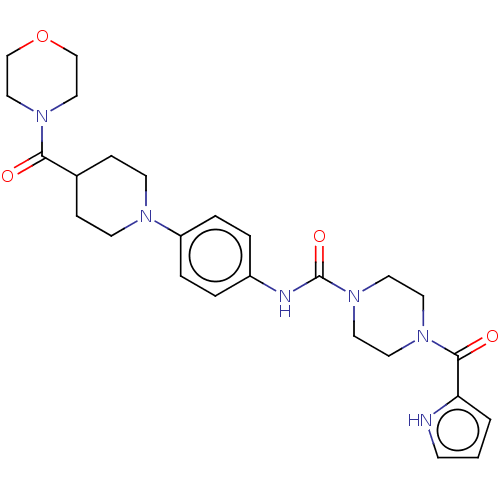 (US8865714, Reference Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136543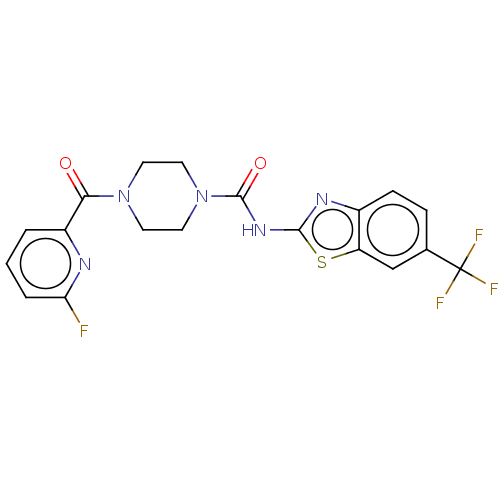 (US8865714, Reference Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136532 (US8865714, Reference Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136539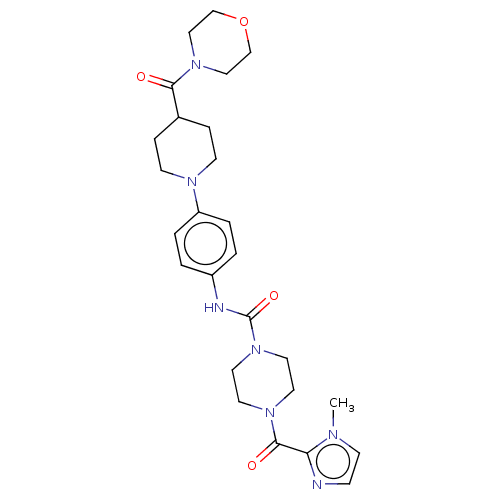 (US8865714, Reference Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136538 (US8865714, Reference Example 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136537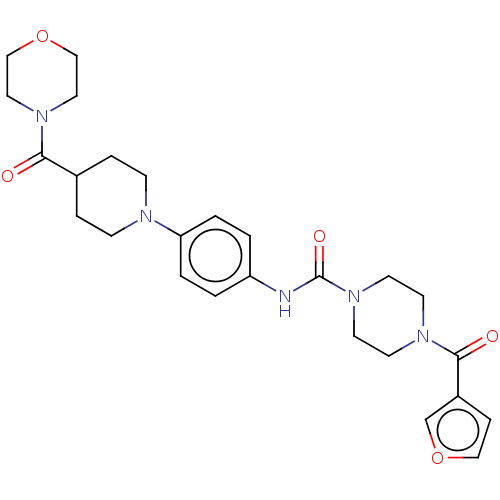 (US8865714, Reference Example 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136536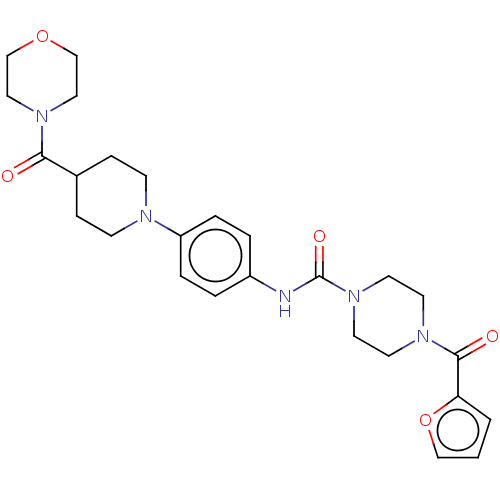 (US8865714, Reference Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136533 (US8865714, Reference Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM136540 (US8865714, Reference Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... | US Patent US8865714 (2014) BindingDB Entry DOI: 10.7270/Q2QF8RKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||