

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142607 (US8933228, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142602 (US8933228, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142601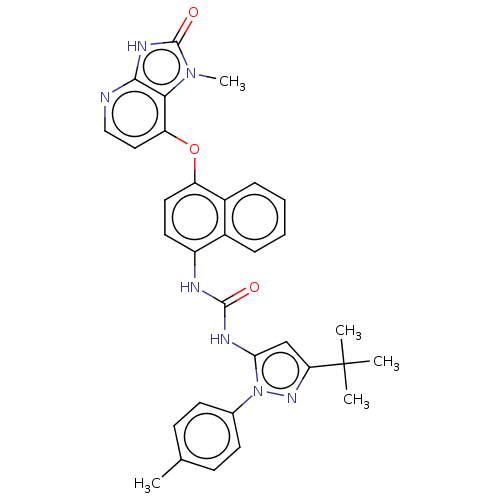 (US8933228, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142627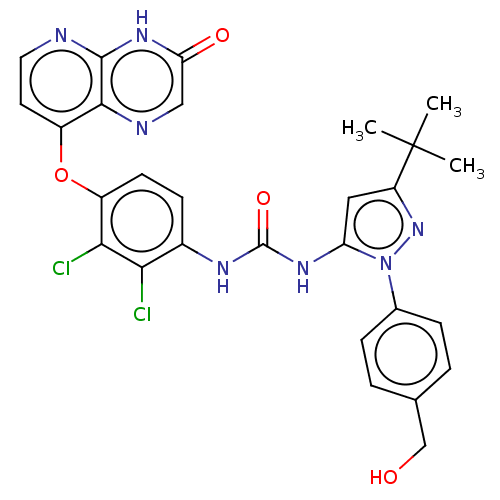 (US8933228, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142603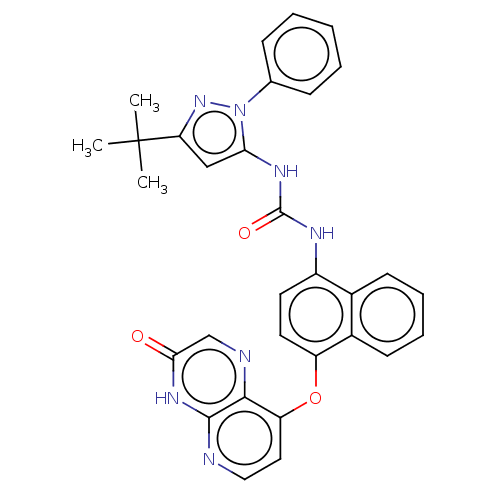 (US8933228, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142607 (US8933228, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142620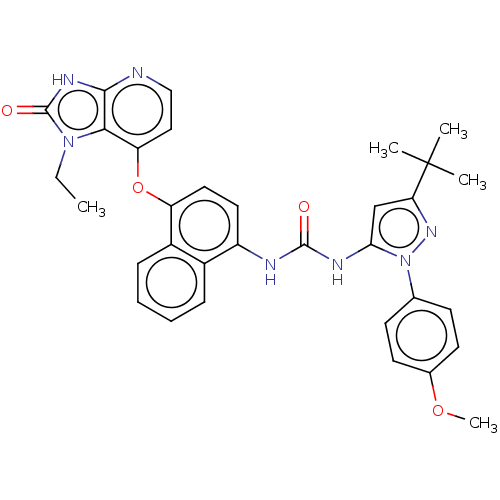 (US8933228, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142618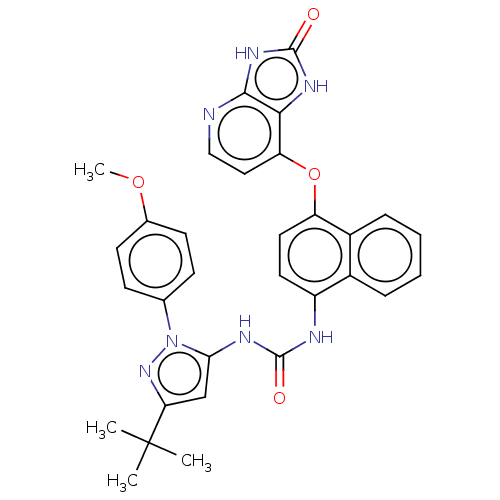 (US8933228, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142601 (US8933228, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142600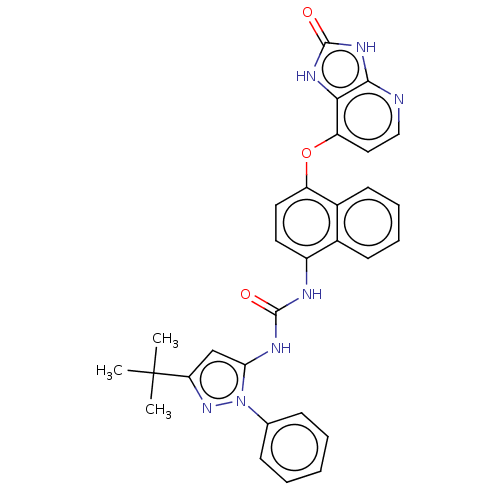 (US8933228, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142599 (US8933228, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142614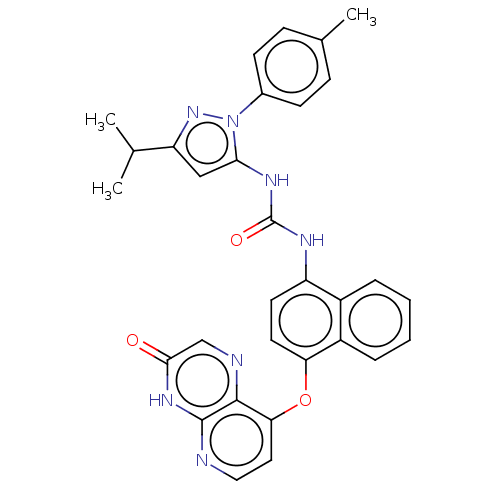 (US8933228, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142612 (US8933228, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142629 (US8933228, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142598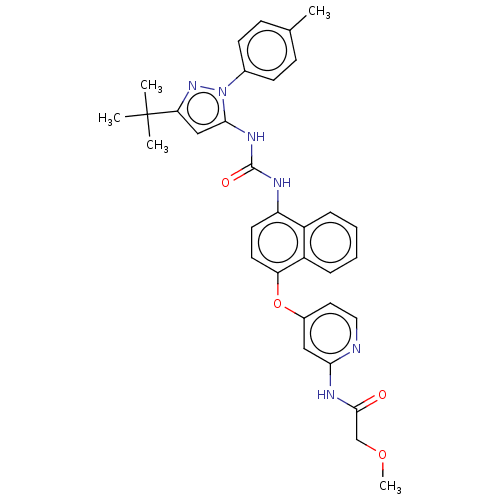 (US10238658, Example Reference | US10813932, Refere...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142610 (US8933228, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142615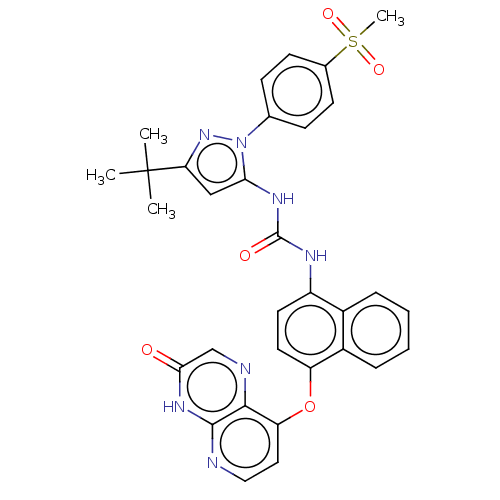 (US8933228, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142605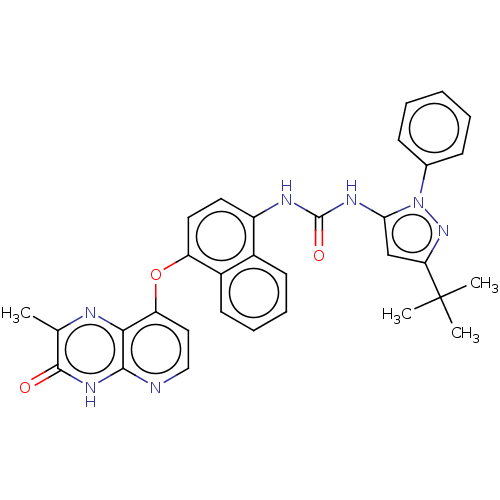 (US8933228, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142629 (US8933228, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142619 (US8933228, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142604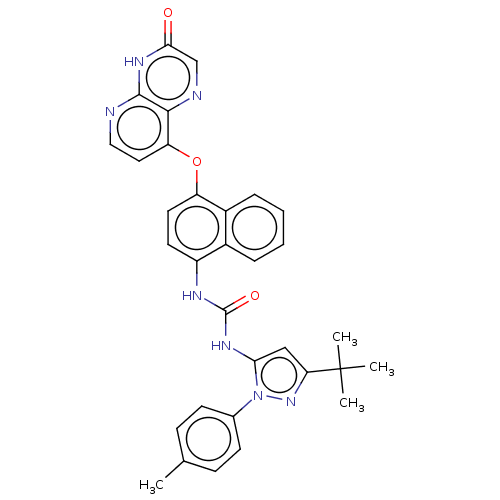 (US8933228, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142604 (US8933228, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142603 (US8933228, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142611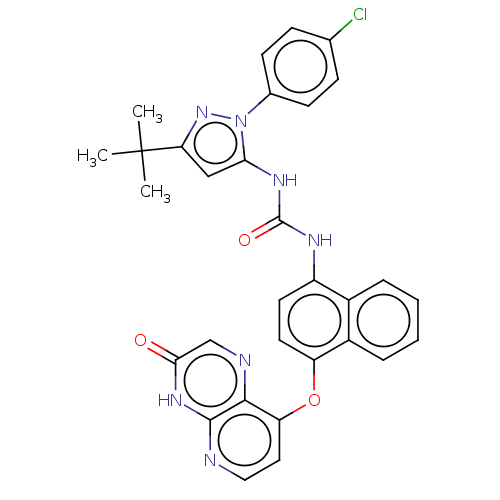 (US8933228, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142605 (US8933228, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142628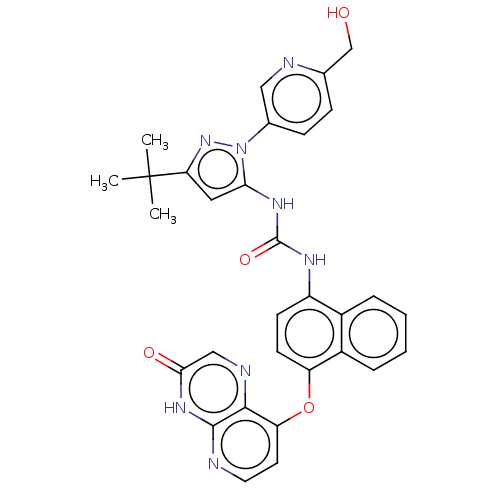 (US8933228, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142613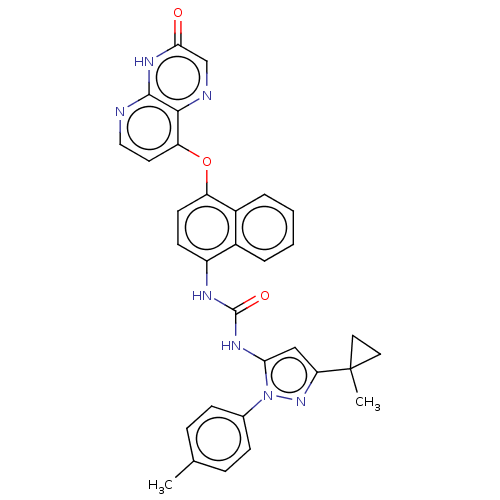 (US8933228, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142624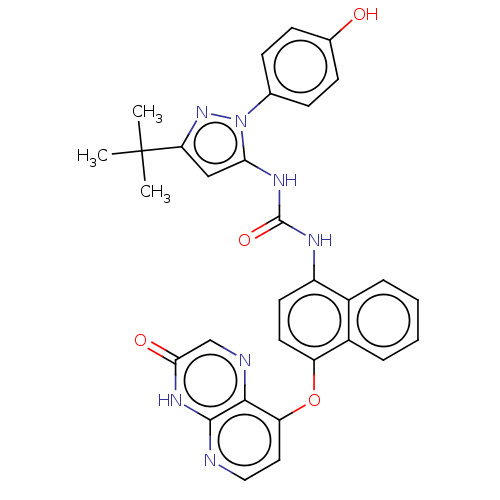 (US8933228, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142627 (US8933228, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142601 (US8933228, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142614 (US8933228, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142617 (US8933228, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142616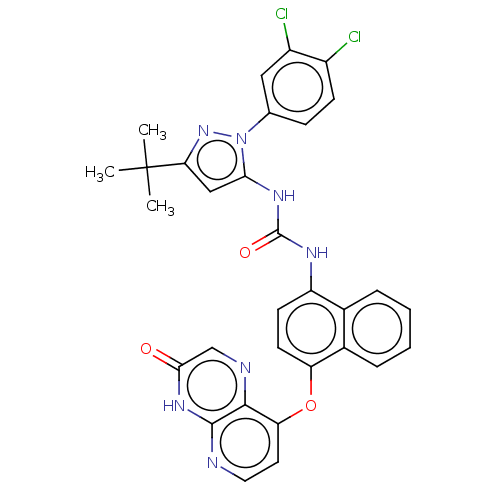 (US8933228, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142616 (US8933228, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142609 (US8933228, 22 | US8933228, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142622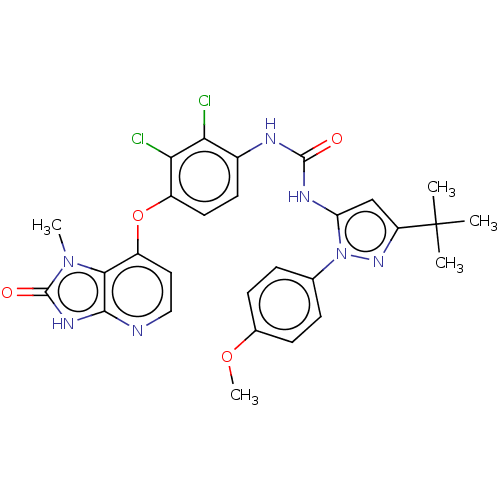 (US8933228, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142598 (US10238658, Example Reference | US10813932, Refere...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142609 (US8933228, 22 | US8933228, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142608 (US8933228, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142626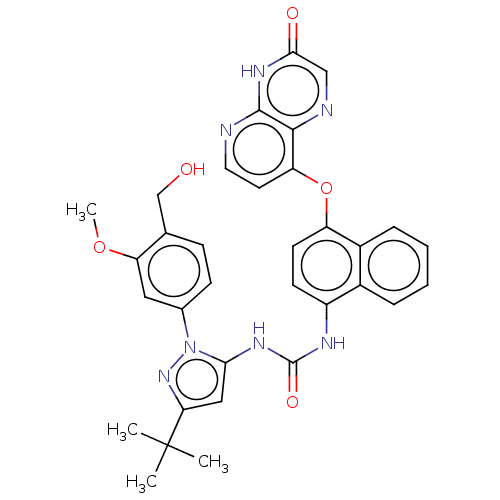 (US8933228, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142620 (US8933228, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142606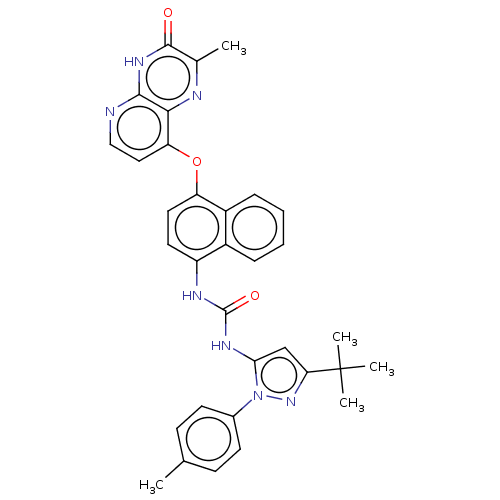 (US8933228, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142617 (US8933228, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142615 (US8933228, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142613 (US8933228, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142626 (US8933228, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142607 (US8933228, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142597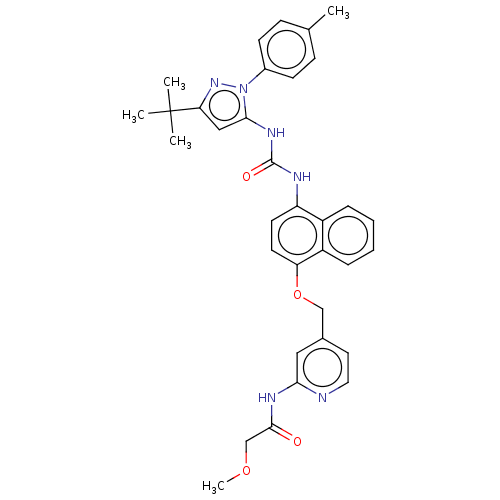 (US8933228, Ref 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142600 (US8933228, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142622 (US8933228, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |