

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM15867 (1-{[(5-chloropyridin-2-yl)carbamoyl]methyl}-N-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15862 (1-{[3-(5-chlorothiophen-2-yl)-1,2-oxazol-5-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372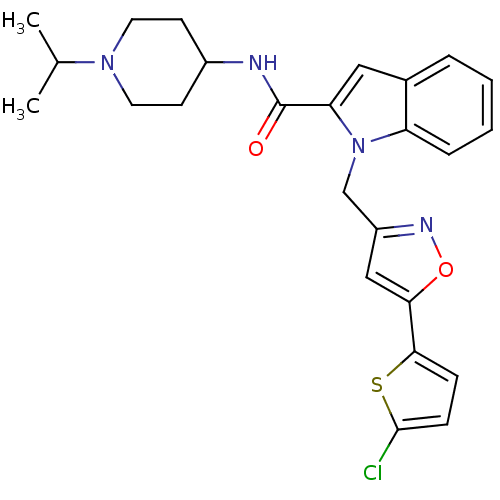 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12400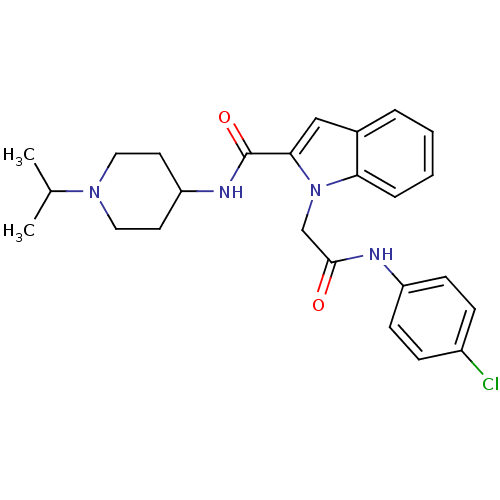 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15863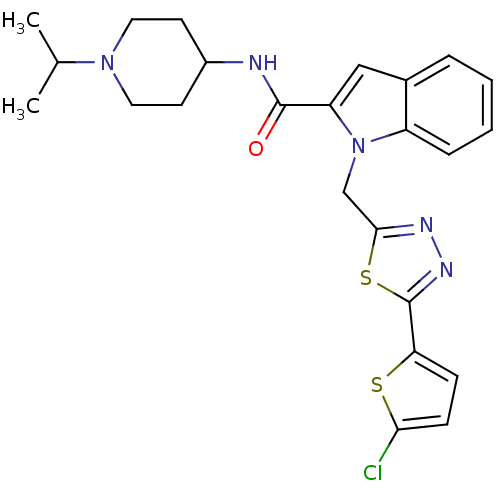 (1-{[5-(5-chlorothiophen-2-yl)-1,3,4-thiadiazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15865 (1-{[(5-chlorothiophen-2-yl)carbamoyl]methyl}-N-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15857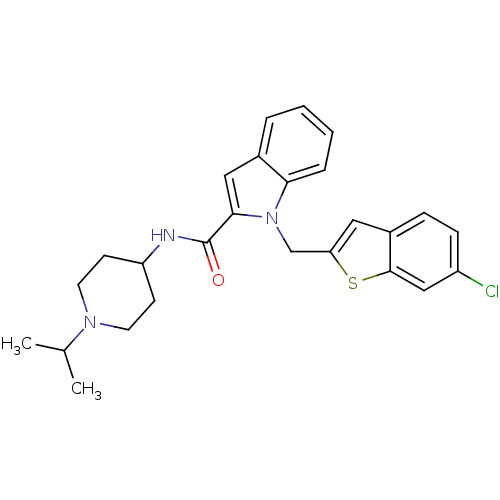 (1-[(6-chloro-1-benzothiophen-2-yl)methyl]-N-[1-(pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15864 (1-{[2-(5-chlorothiophen-2-yl)-1,3-thiazol-5-yl]met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15854 (1-[2-(4-chlorophenyl)ethyl]-N-[1-(propan-2-yl)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12398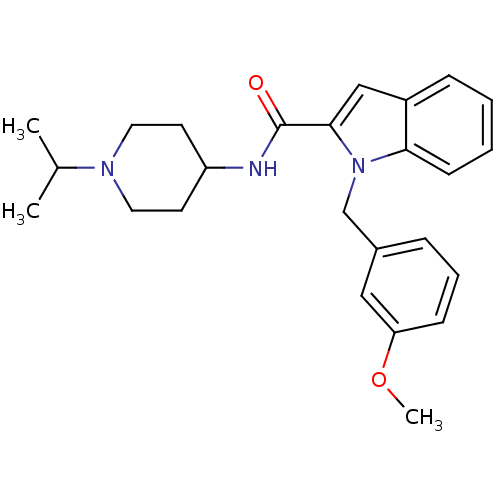 (1-(3-Methoxy-benzyl)-1H-indole-2-carboxylic acid (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 89 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15866 (1-{[(3-chlorophenyl)carbamoyl]methyl}-N-[1-(propan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 287 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15859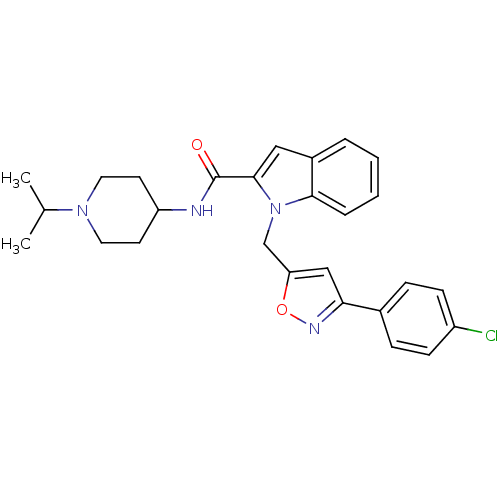 (1-{[3-(4-chlorophenyl)-1,2-oxazol-5-yl]methyl}-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15855 (1-[2-(2,4-dichlorophenyl)ethyl]-N-[1-(propan-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 474 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15853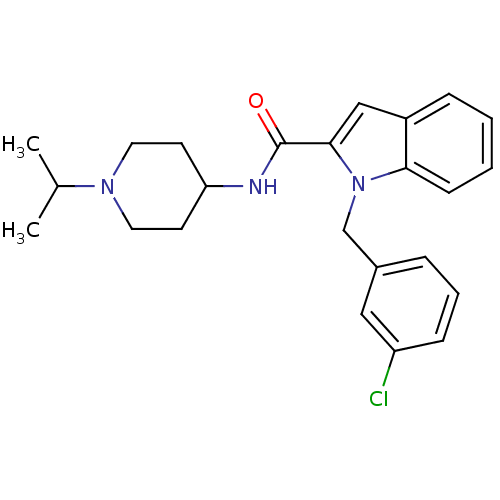 (1-[(3-chlorophenyl)methyl]-N-[1-(propan-2-yl)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 654 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15858 (1-{[5-(4-chlorophenyl)-1,2-oxazol-3-yl]methyl}-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 810 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15849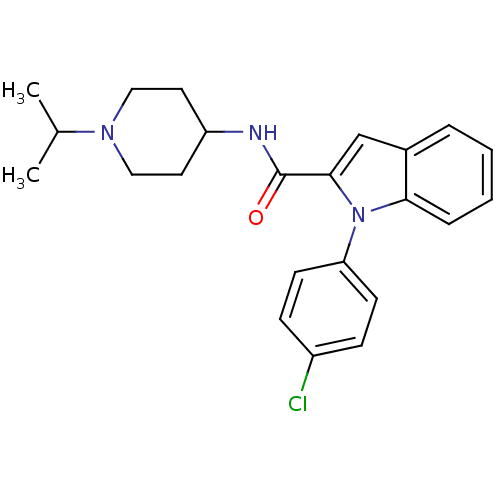 (1-(4-chlorophenyl)-N-[1-(propan-2-yl)piperidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15860 (1-{[3-(4-chlorophenyl)-1,2,4-oxadiazol-5-yl]methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 2.23E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15850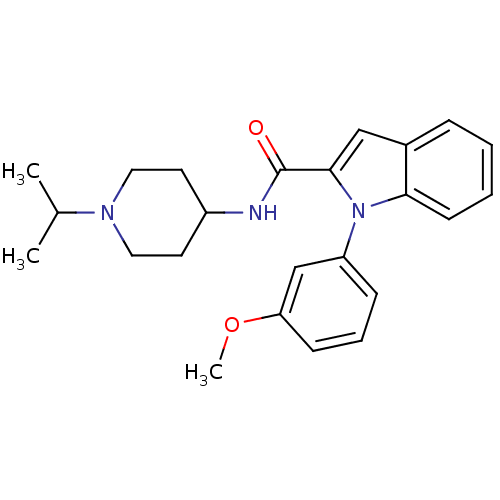 (1-(3-methoxyphenyl)-N-[1-(propan-2-yl)piperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.48E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15852 (1-[(4-chlorophenyl)methyl]-N-[1-(propan-2-yl)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15861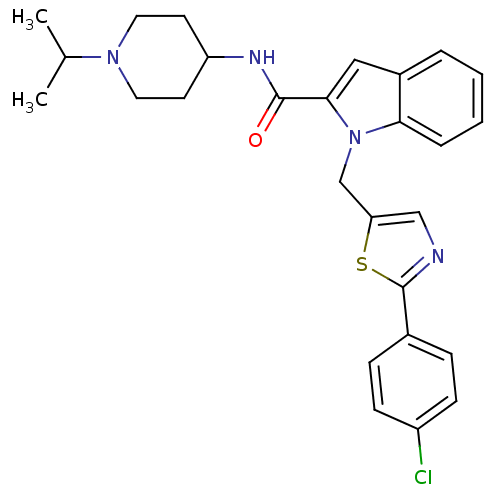 (1-{[2-(4-chlorophenyl)-1,3-thiazol-5-yl]methyl}-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.73E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12372 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.76E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human thrombin was determined by using the chromogenic substrates S-2366. The hydrolysis rates of chromoge... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15851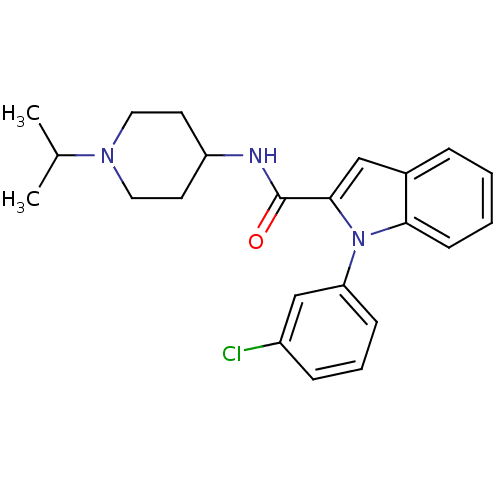 (1-(3-chlorophenyl)-N-[1-(propan-2-yl)piperidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.01E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM15857 (1-[(6-chloro-1-benzothiophen-2-yl)methyl]-N-[1-(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 9.04E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human thrombin was determined by using the chromogenic substrates S-2366. The hydrolysis rates of chromoge... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15856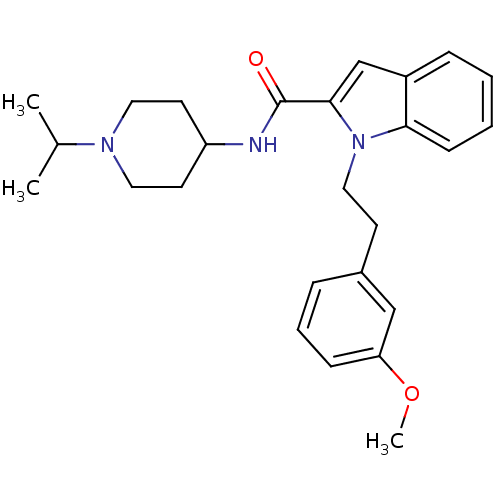 (1-[2-(3-methoxyphenyl)ethyl]-N-[1-(propan-2-yl)pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM15867 (1-{[(5-chloropyridin-2-yl)carbamoyl]methyl}-N-[1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human thrombin was determined by using the chromogenic substrates S-2366. The hydrolysis rates of chromoge... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12400 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human thrombin was determined by using the chromogenic substrates S-2366. The hydrolysis rates of chromoge... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||