

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2606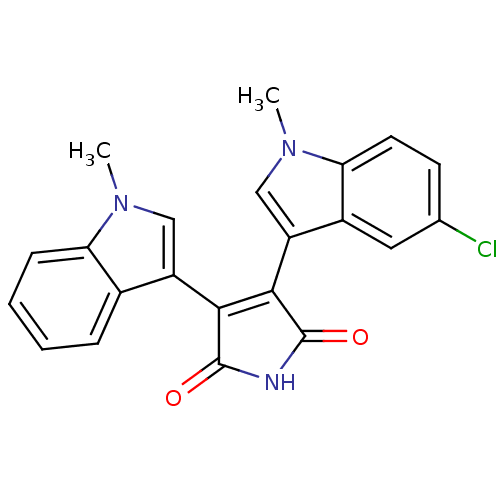 (3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(1-methyl-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2607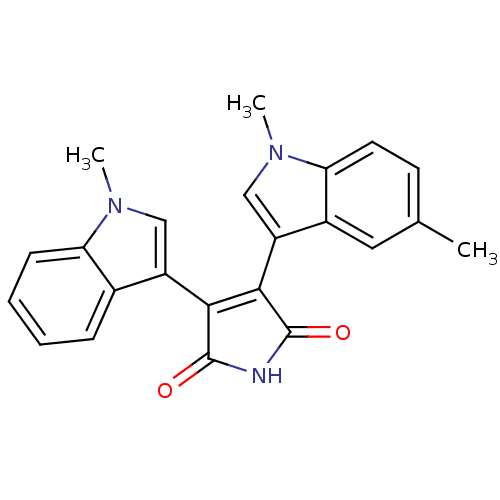 (3-(1,5-dimethyl-1H-indol-3-yl)-4-(1-methyl-1H-indo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2590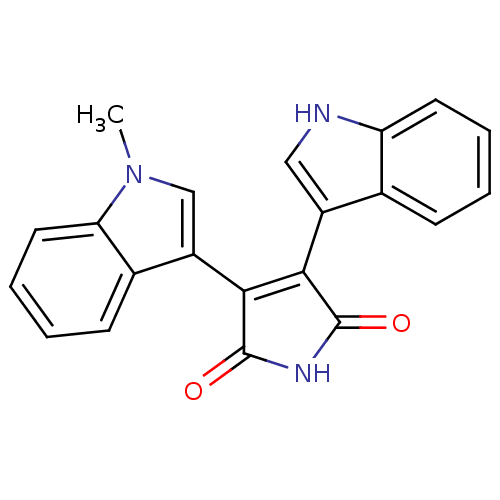 ((Arylindolyl)maleimide deriv. 12 | 3-(1H-indol-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2626 (3-(1,7-dimethyl-1H-indol-3-yl)-4-(1-methyl-1H-indo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2591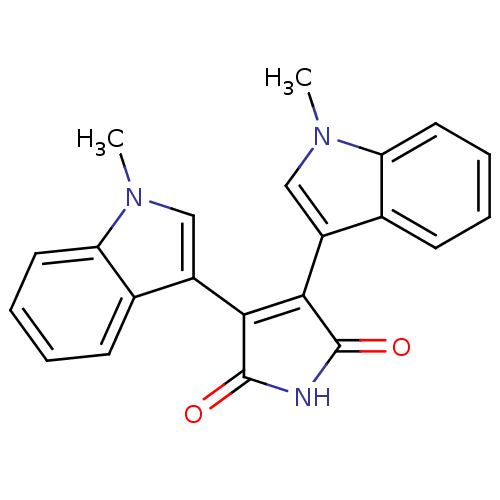 ((Arylindolyl)maleimide deriv. 13 | 3,4-bis(1-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2620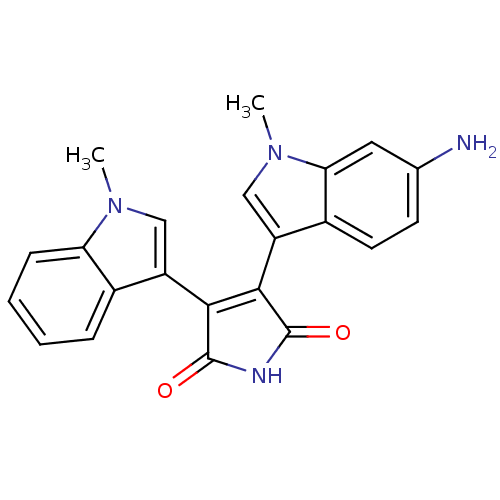 (3-(6-amino-1-methyl-1H-indol-3-yl)-4-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2594 ((Arylindolyl)maleimide deriv. 16 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2612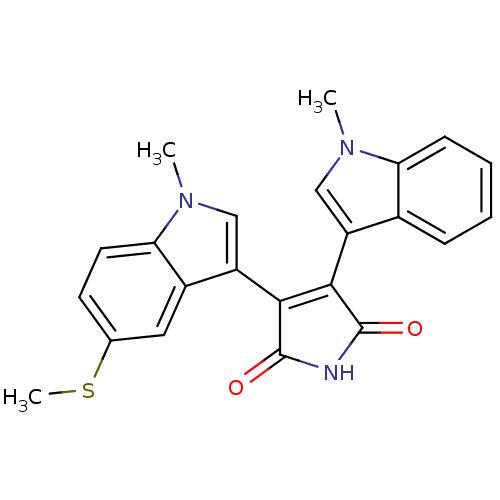 (3-(1-methyl-1H-indol-3-yl)-4-[1-methyl-5-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2635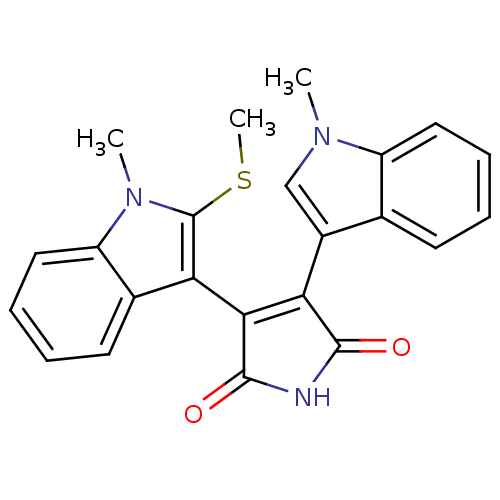 (3-(1-methyl-1H-indol-3-yl)-4-[1-methyl-2-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2634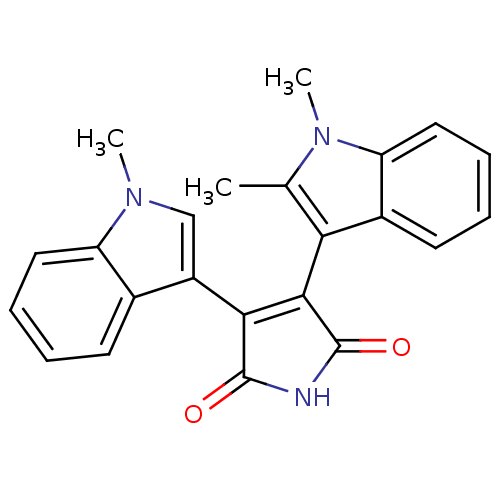 (3-(1,2-dimethyl-1H-indol-3-yl)-4-(1-methyl-1H-indo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2580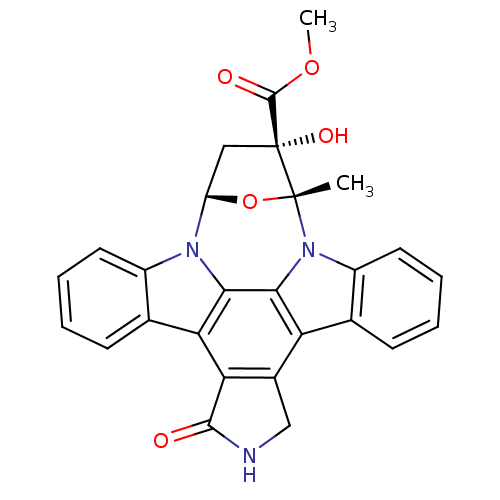 (K252a | methyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2593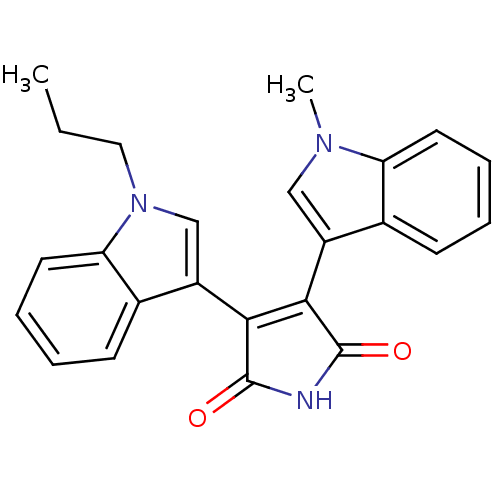 ((Arylindolyl)maleimide deriv. 15 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2608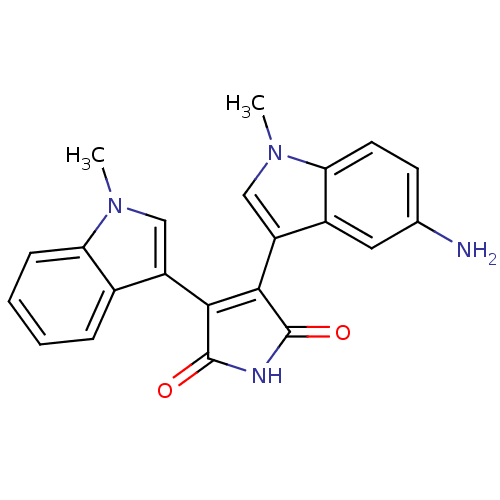 (3-(5-amino-1-methyl-1H-indol-3-yl)-4-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2583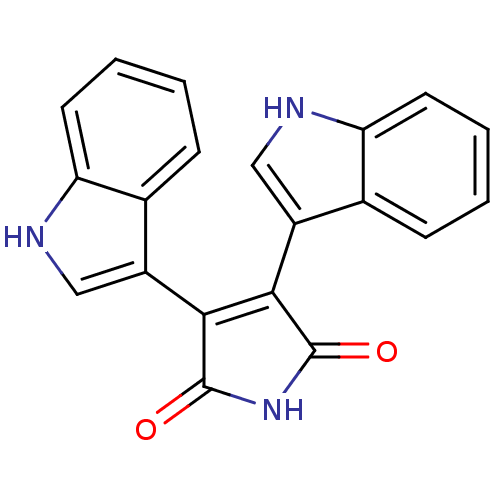 (3,4-Bis(3-indolyl)-1H-pyrrole-2,5-dione | 3,4-bis(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2631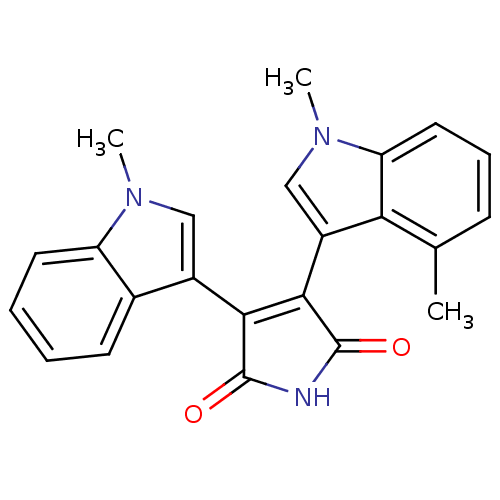 (3-(1,4-dimethyl-1H-indol-3-yl)-4-(1-methyl-1H-indo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2610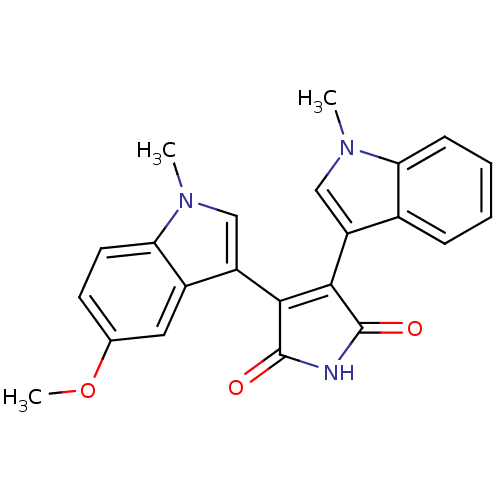 (3-(5-Methoxy-1-methyl-3-indolyl)-4-(1-methyl-3-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2652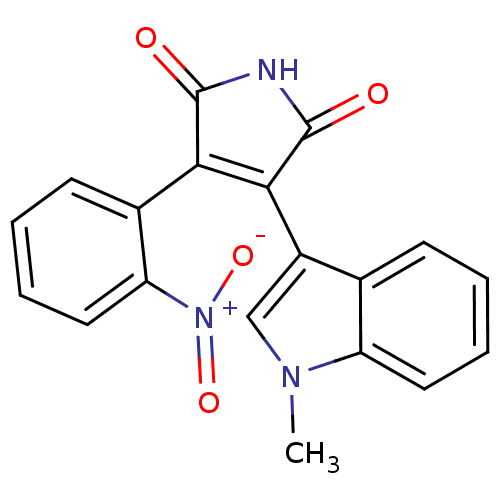 ((Phenylindolyl)maleimide deriv. 74 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2597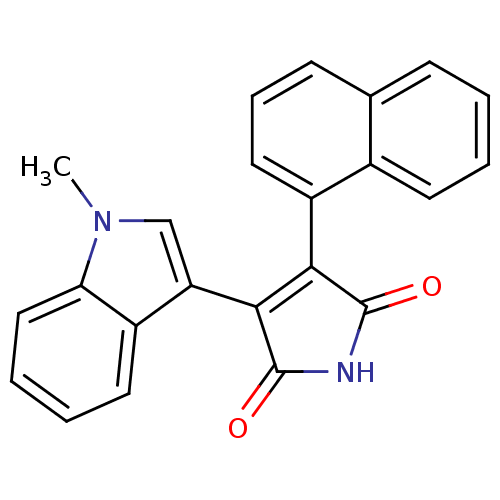 ((Arylindolyl)maleimide deriv. 19 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2651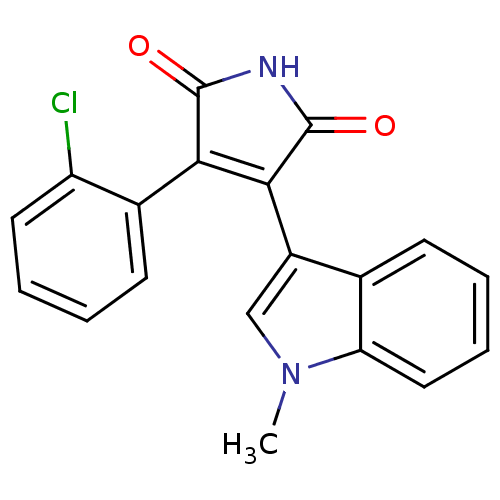 ((Phenylindolyl)maleimide deriv. 73 | 3-(2-chloroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2599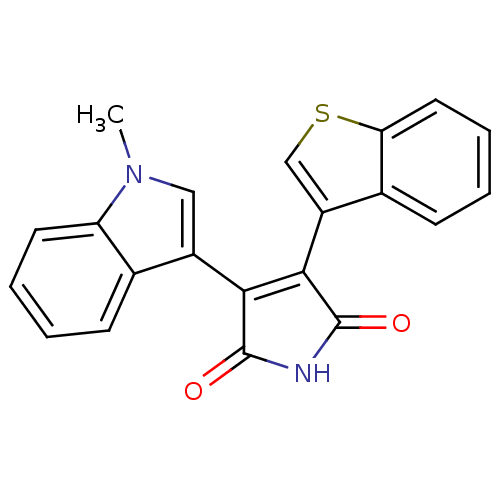 ((Arylindolyl)maleimide deriv. 21 | 3-(1-benzothiop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2609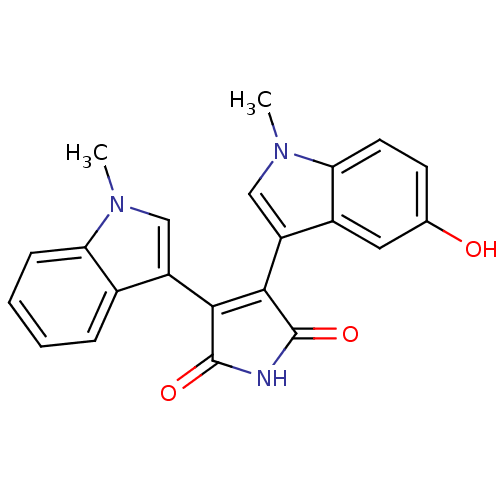 (3-(5-Hydroxy-1-methyl-3-indolyl)-4-(1-methyl-3-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2653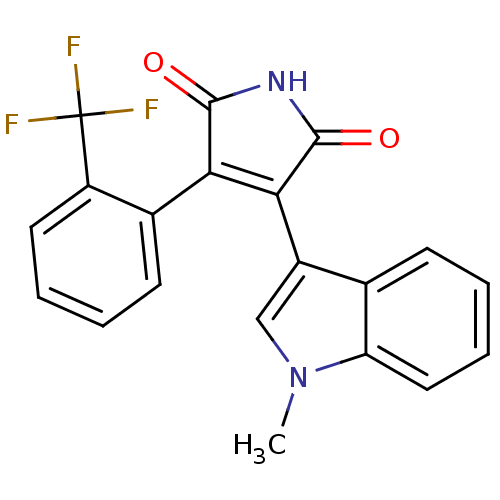 ((Phenylindolyl)maleimide deriv. 75 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2627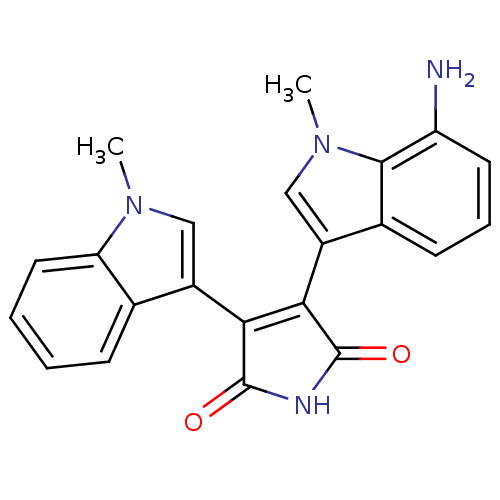 (3-(7-Amino-1-methyl-3-indolyl)-4-(1-methyl-3-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2628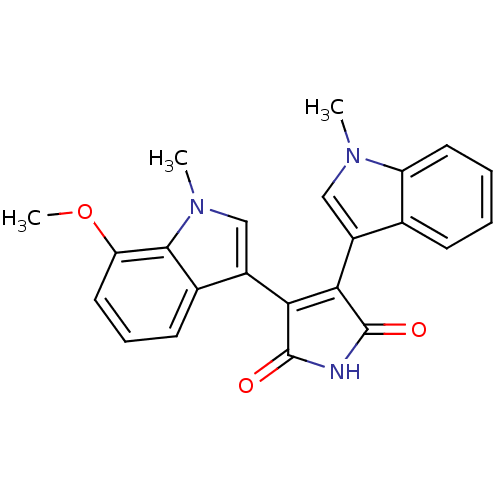 (3-(7-methoxy-1-methyl-1H-indol-3-yl)-4-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2658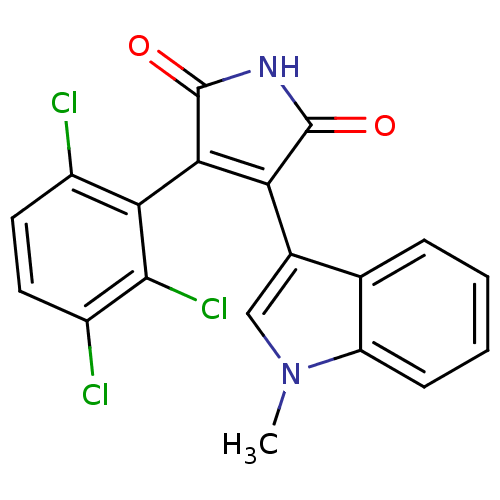 ((Phenylindolyl)maleimide deriv. 80 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2642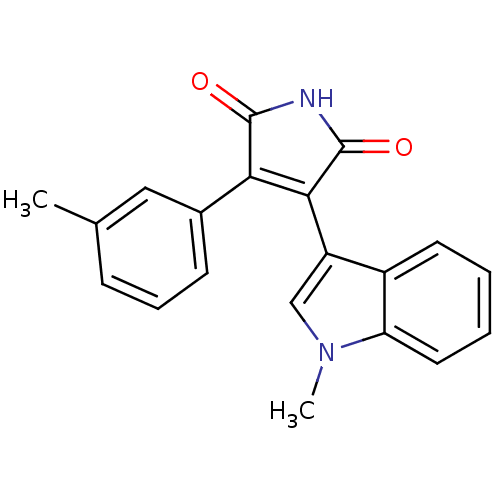 ((Phenylindolyl)maleimide deriv. 64 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2636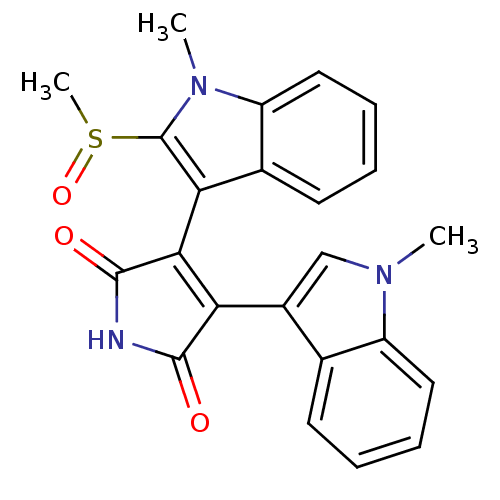 (3-(2-methanesulfinyl-1-methyl-1H-indol-3-yl)-4-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2622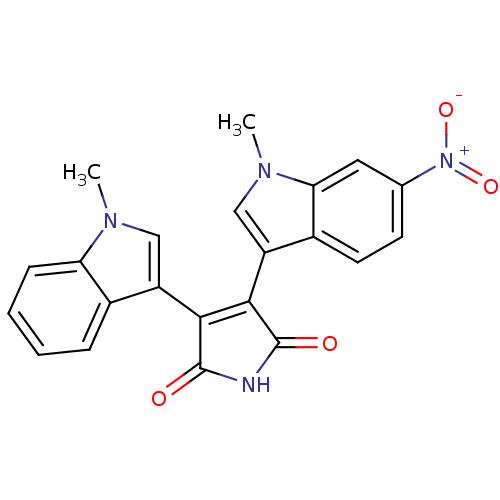 (3-(1-methyl-1H-indol-3-yl)-4-(1-methyl-6-nitro-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2621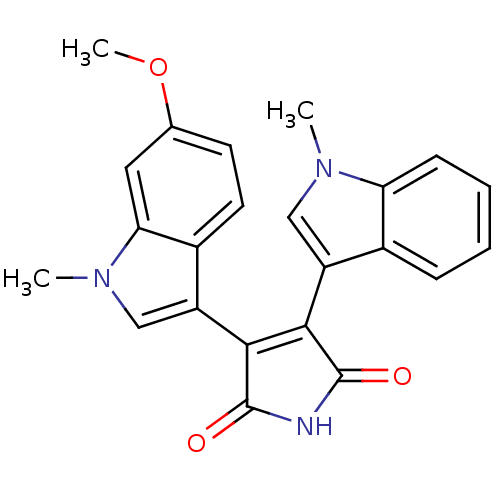 (3-(6-methoxy-1-methyl-1H-indol-3-yl)-4-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2655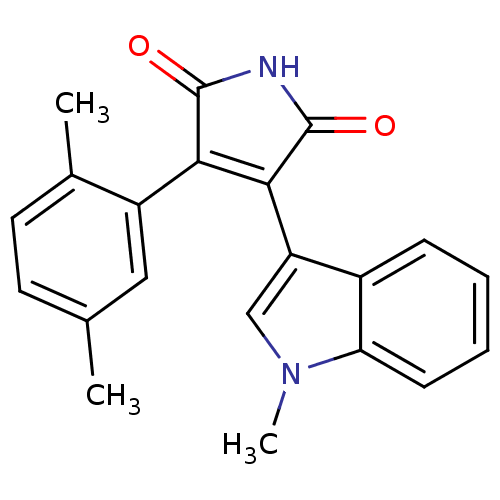 ((Phenylindolyl)maleimide deriv. 77 | 3-(2,5-dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2592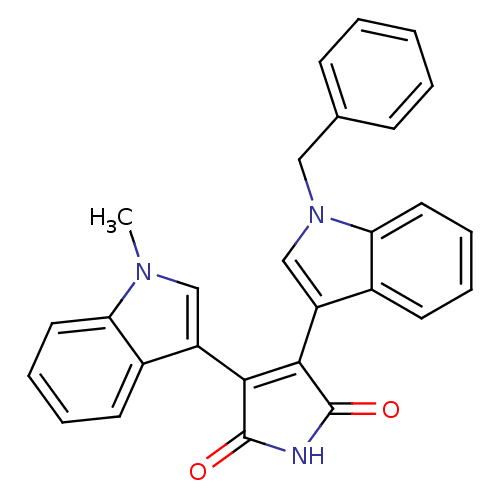 ((Arylindolyl)maleimide deriv. 14 | 3-(1-benzyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2615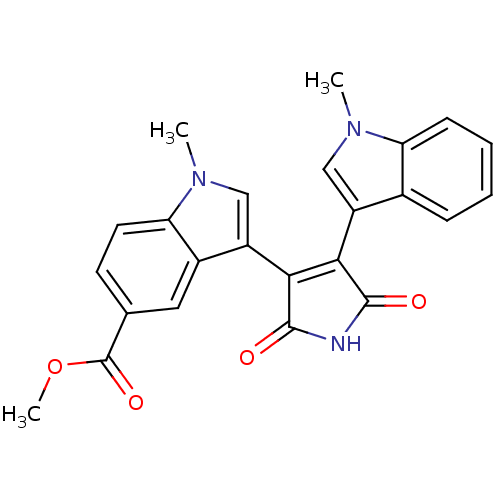 (Bisindolylmaleimide deriv. 37 | methyl 1-methyl-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2629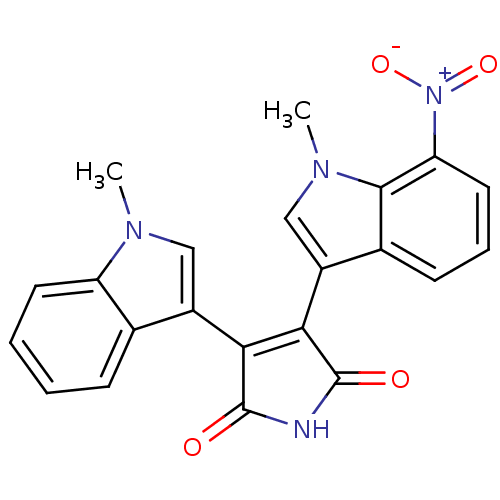 (3-(1-Methyl-3-indolyl)-4-(1-methyl-7-nitro-3-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2619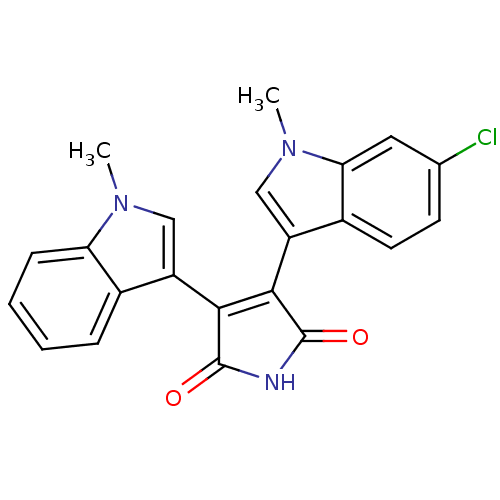 (3-(6-chloro-1-methyl-1H-indol-3-yl)-4-(1-methyl-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2648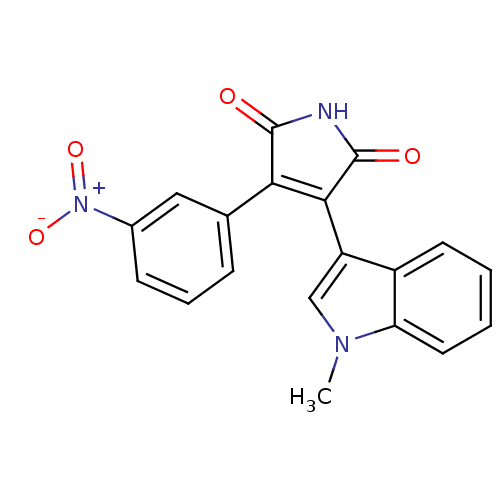 ((Phenylindolyl)maleimide deriv. 70 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2643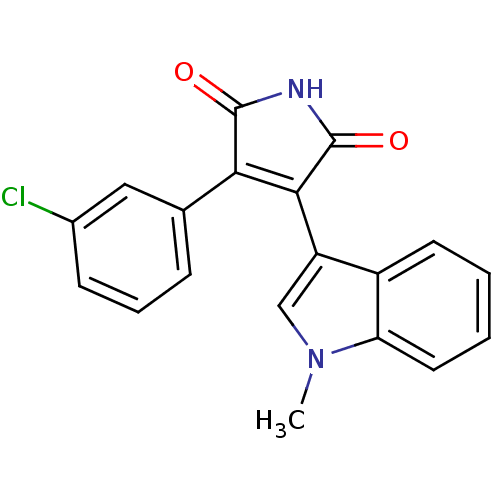 ((Phenylindolyl)maleimide deriv. 65 | 3-(3-chloroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2633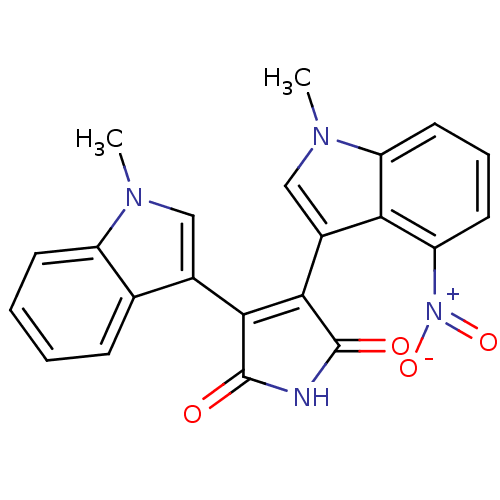 (3-(1-methyl-1H-indol-3-yl)-4-(1-methyl-4-nitro-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2650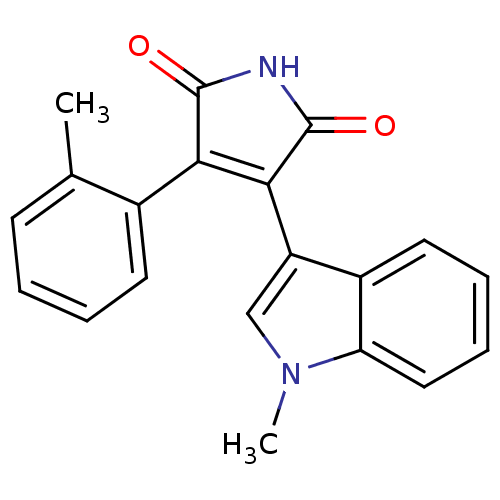 ((Phenylindolyl)maleimide deriv. 72 | 3-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2644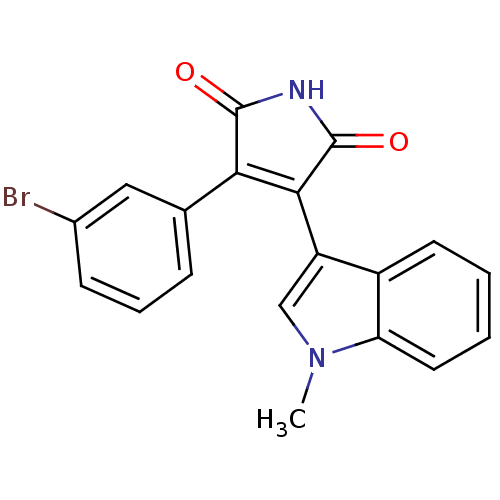 ((Phenylindolyl)maleimide deriv. 66 | 3-(3-bromophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2630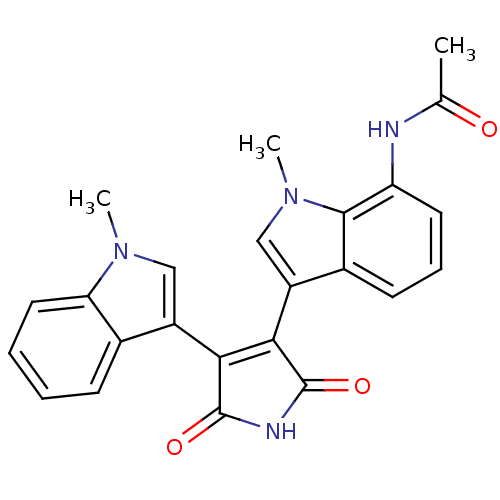 (3-(7-(Acetylamino)-1-methyl-3-indolyl)-4-(1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2656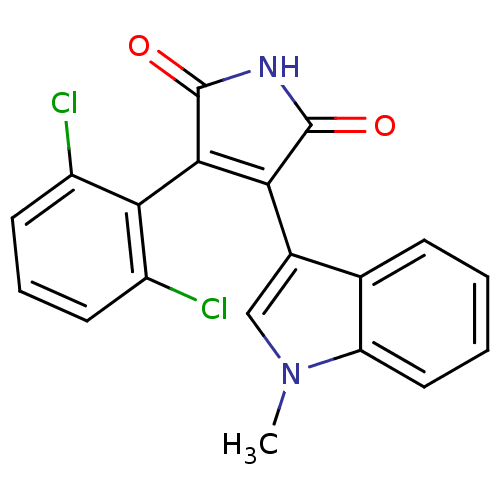 ((Phenylindolyl)maleimide deriv. 78 | 3-(2,6-dichlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2654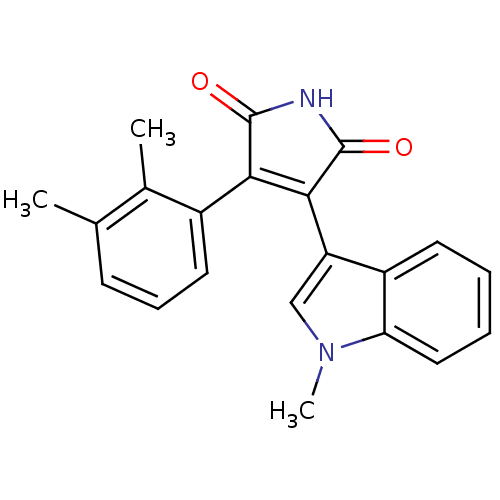 ((Phenylindolyl)maleimide deriv. 76 | 3-(2,3-dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2614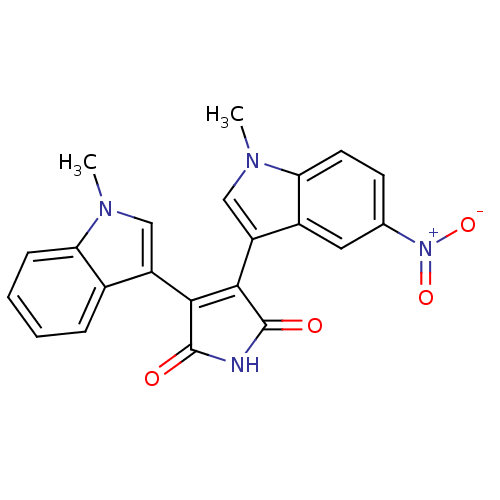 (3-(1-methyl-1H-indol-3-yl)-4-(1-methyl-5-nitro-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2600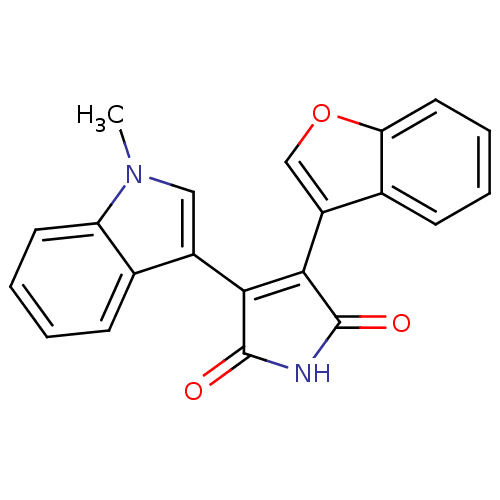 ((Arylindolyl)maleimide deriv. 22 | 3-(1-benzofuran...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2598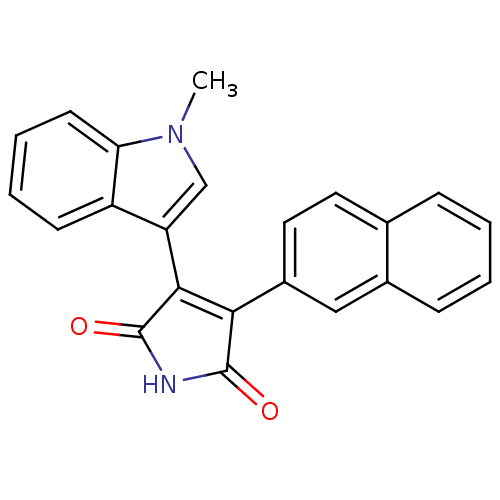 ((Arylindolyl)maleimide deriv. 20 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2605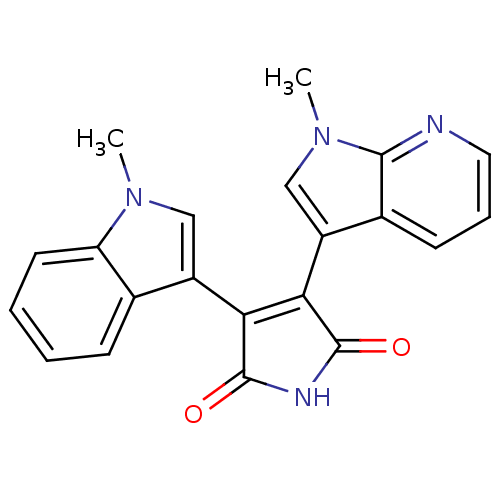 ((Arylindolyl)maleimide deriv. 27 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2596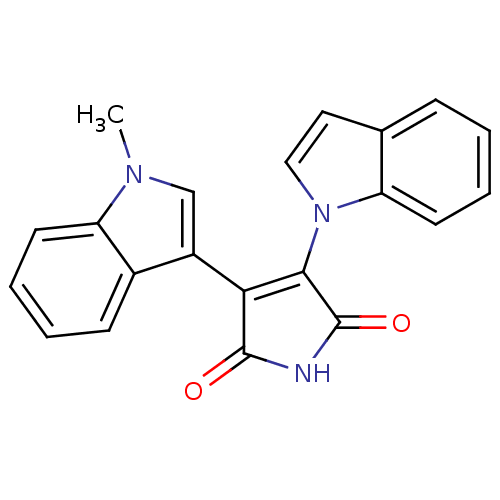 ((Arylindolyl)maleimide deriv. 18 | 3-(1H-indol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2613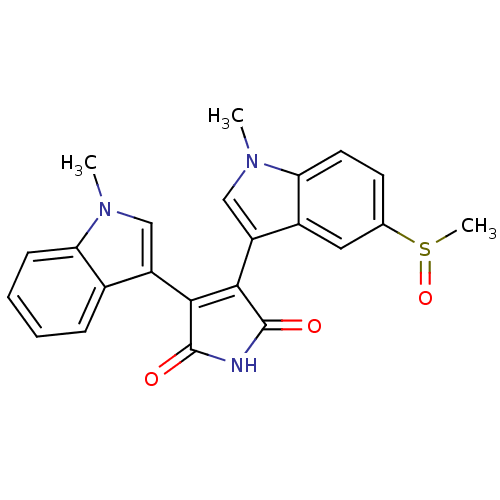 (3-(1-Methyl-3-indolyl)-4-(1-methyl-5-(methylsulfin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2632 (3-(4-methoxy-1-methyl-1H-indol-3-yl)-4-(1-methyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |