

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM19770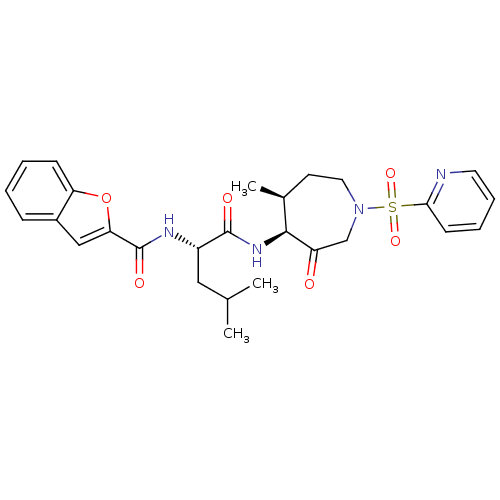 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775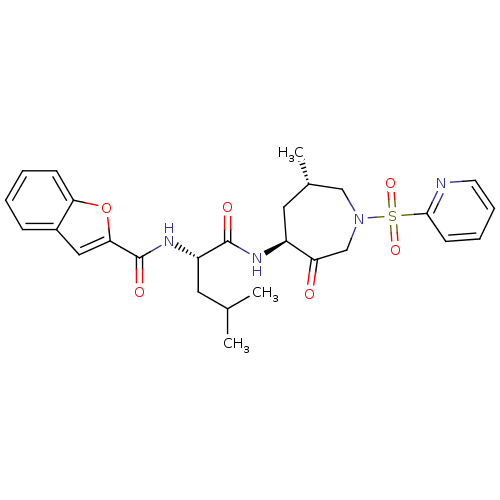 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19780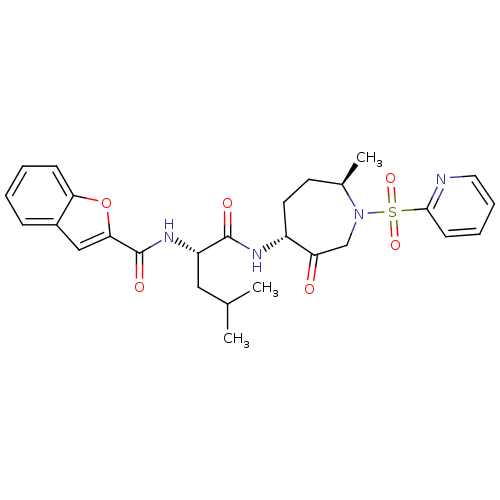 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19771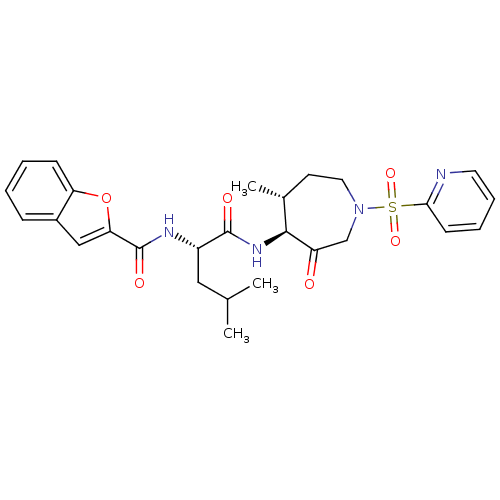 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19779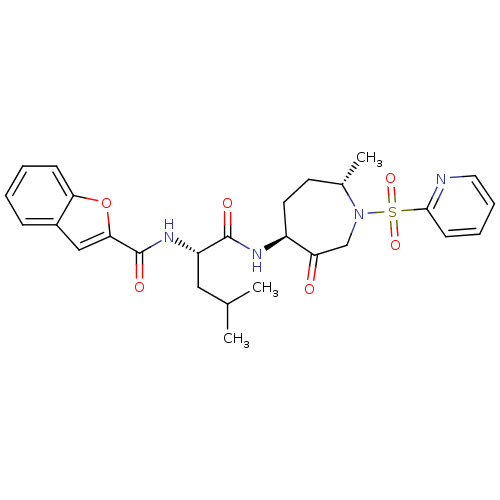 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -48.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19781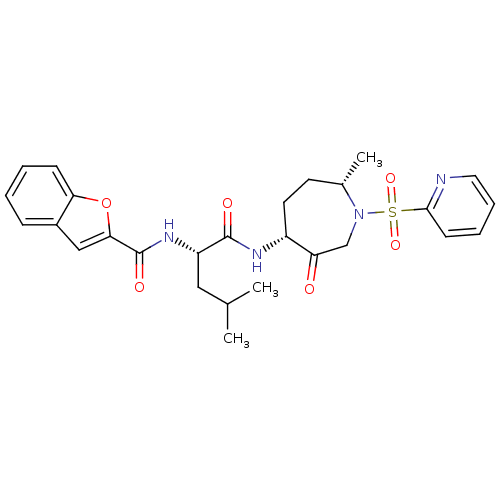 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19781 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19771 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19777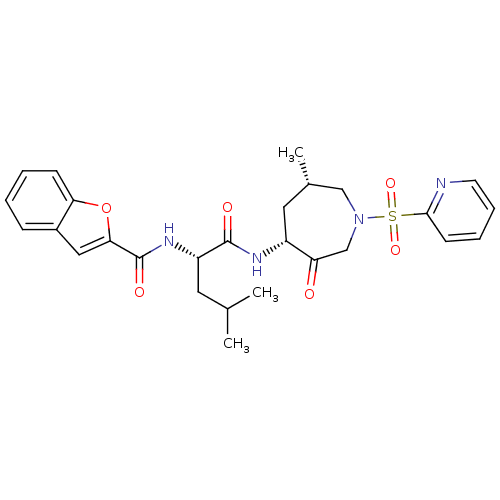 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19780 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | -47.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19779 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | -45.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19777 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19777 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19772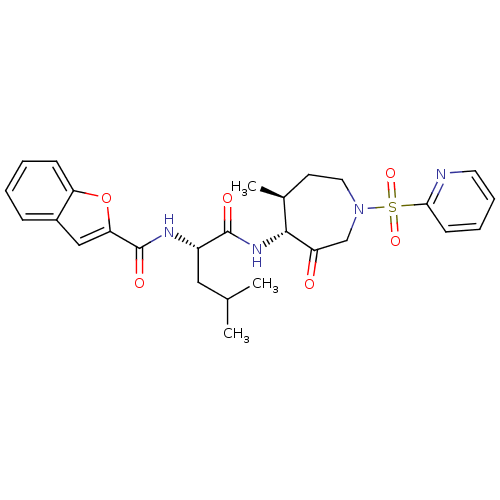 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19774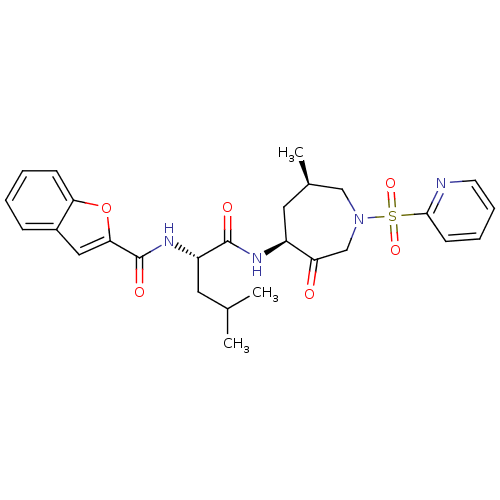 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19776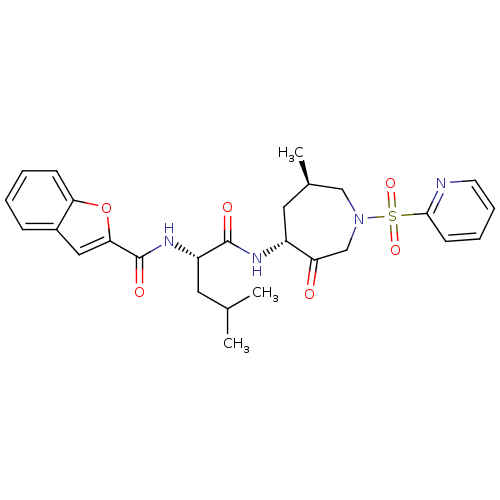 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19779 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19773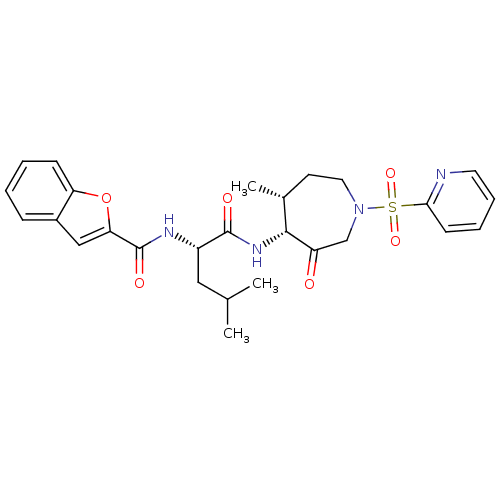 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | -41.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19773 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19772 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19780 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19781 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19776 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19774 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19776 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19774 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19780 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19777 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19779 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19781 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19771 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19773 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM19774 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |