

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142598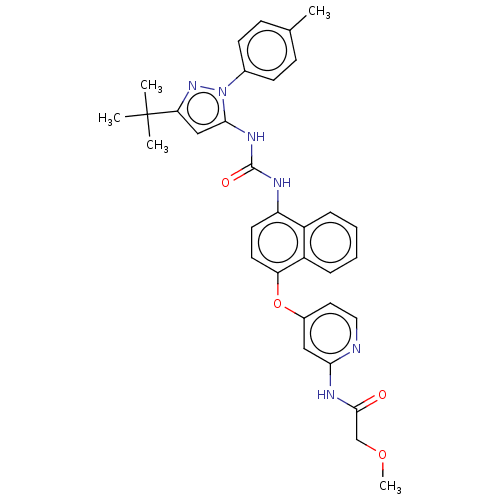 (US10238658, Example Reference | US10813932, Refere...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of compound was determined by FRET using synthetic peptides labelled with both donor and acceptor fluorophores (Z-LY... | US Patent US9242960 (2016) BindingDB Entry DOI: 10.7270/Q20K27C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of compound was determined by FRET using synthetic peptides labelled with both donor and acceptor fluorophores (Z-LY... | US Patent US9242960 (2016) BindingDB Entry DOI: 10.7270/Q20K27C2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 12 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | 25 |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of compound was determined by FRET using synthetic peptides labelled with both donor and acceptor fluorophores (Z-LY... | US Patent US9242960 (2016) BindingDB Entry DOI: 10.7270/Q20K27C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 12 (Homo sapiens (Human)) | BDBM142598 (US10238658, Example Reference | US10813932, Refere...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | 25 |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of compound was determined by FRET using synthetic peptides labelled with both donor and acceptor fluorophores (Z-LY... | US Patent US9242960 (2016) BindingDB Entry DOI: 10.7270/Q20K27C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||