

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21266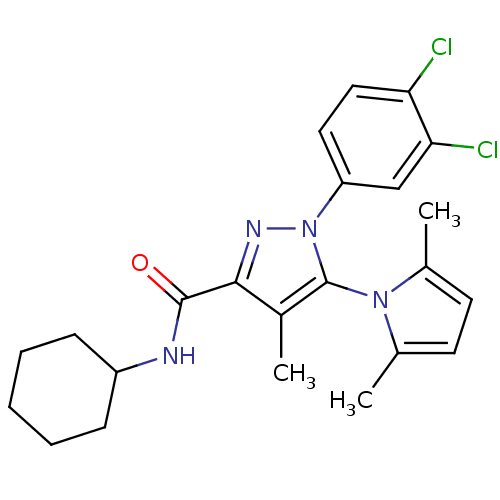 (N-cyclohexyl-1-(3,4-dichlorophenyl)-5-(2,5-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21263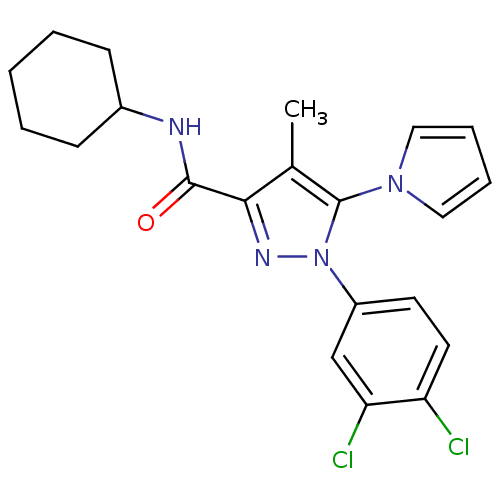 (N-cyclohexyl-1-(3,4-dichlorophenyl)-4-methyl-5-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21279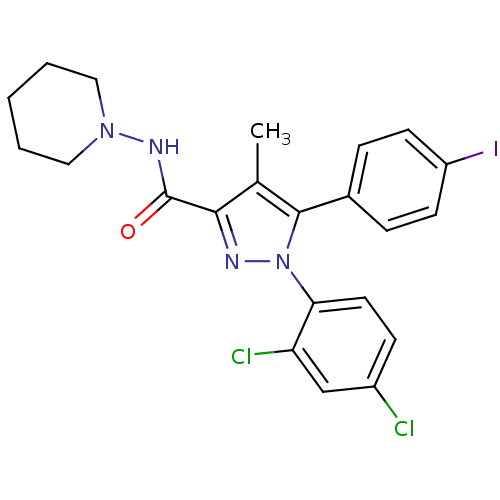 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21280 (5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21263 (N-cyclohexyl-1-(3,4-dichlorophenyl)-4-methyl-5-(1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21266 (N-cyclohexyl-1-(3,4-dichlorophenyl)-5-(2,5-dimethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21261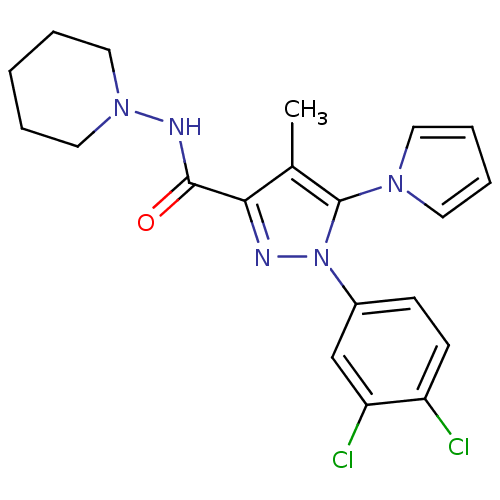 (1-(3,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21259 (1-(2,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21264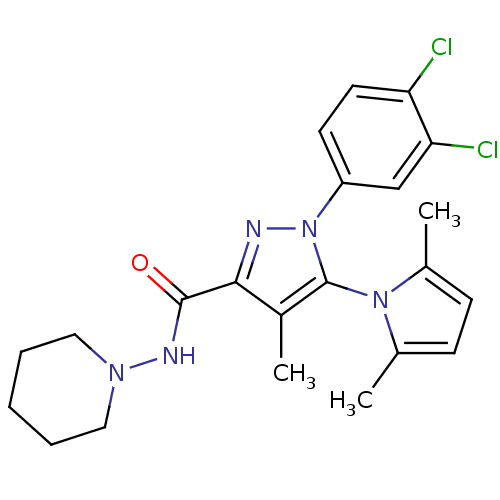 (1-(3,4-dichlorophenyl)-5-(2,5-dimethyl-1H-pyrrol-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21277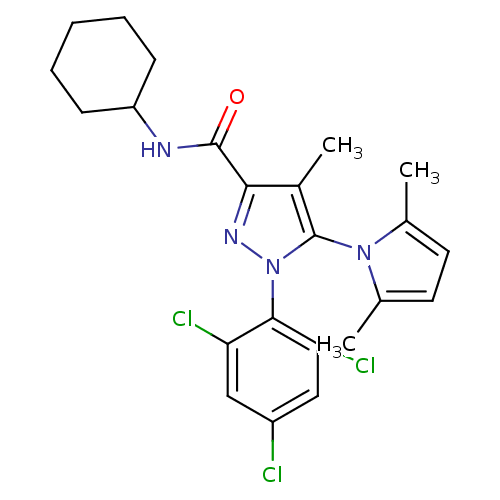 (N-cyclohexyl-5-(2,5-dimethyl-1H-pyrrol-1-yl)-4-met...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21264 (1-(3,4-dichlorophenyl)-5-(2,5-dimethyl-1H-pyrrol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21261 (1-(3,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21272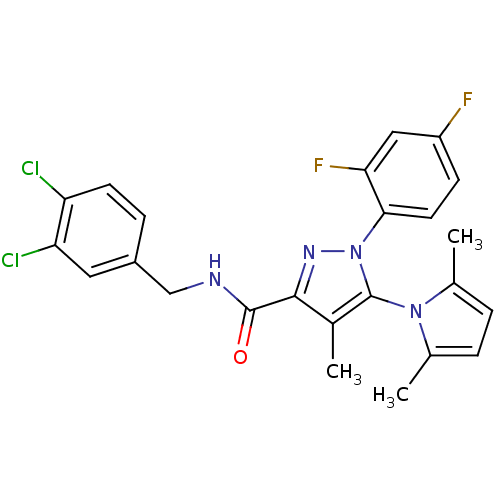 (N-[(3,4-dichlorophenyl)methyl]-1-(2,4-difluorophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21273 (N-cyclohexyl-1-(2,4-difluorophenyl)-5-(2,5-dimethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21262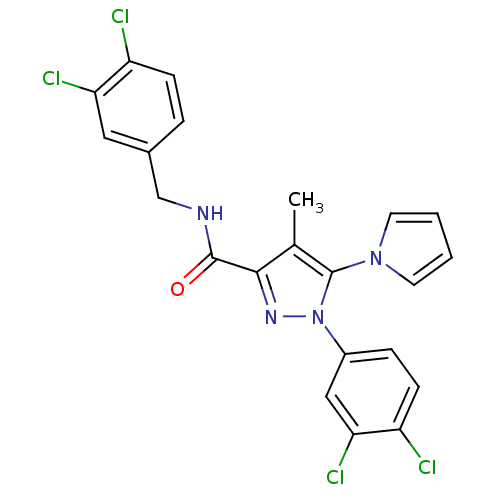 (1-(3,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21256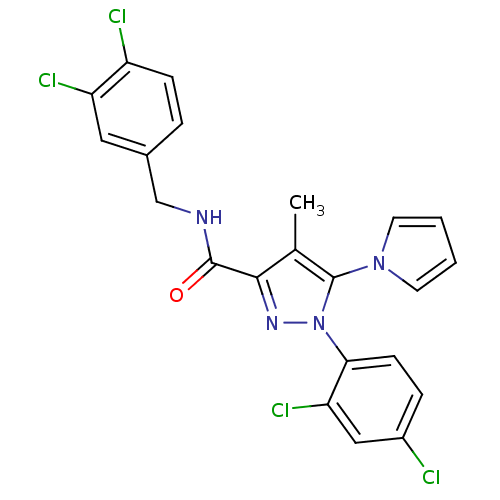 (1-(2,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21258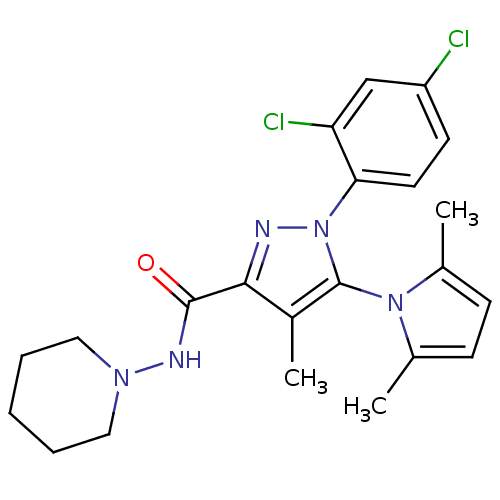 (1-(2,4-dichlorophenyl)-5-(2,5-dimethyl-1H-pyrrol-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21260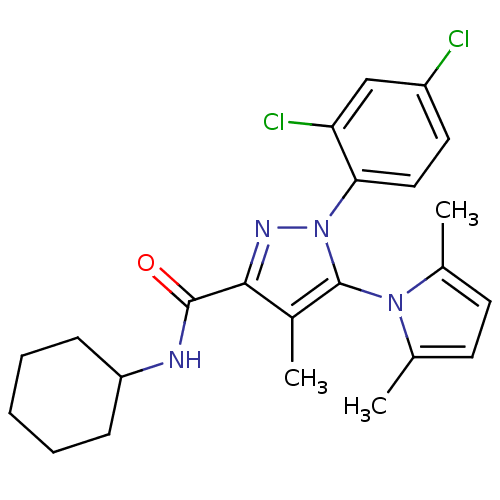 (N-cyclohexyl-1-(2,4-dichlorophenyl)-5-(2,5-dimethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21257 (N-cyclohexyl-1-(2,4-dichlorophenyl)-4-methyl-5-(1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21257 (N-cyclohexyl-1-(2,4-dichlorophenyl)-4-methyl-5-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21270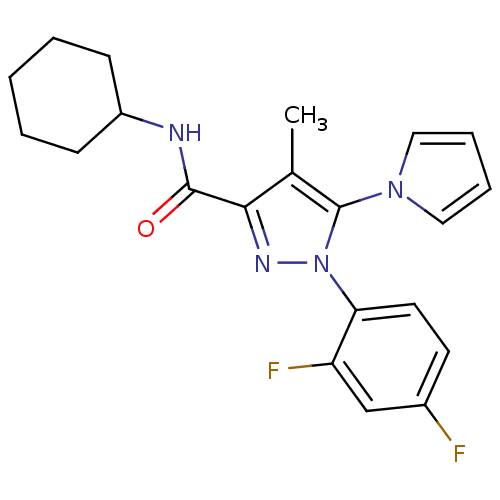 (N-cyclohexyl-1-(2,4-difluorophenyl)-4-methyl-5-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21260 (N-cyclohexyl-1-(2,4-dichlorophenyl)-5-(2,5-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21251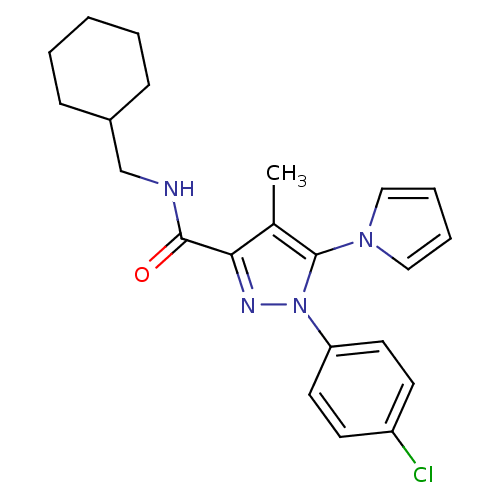 (1-(4-chlorophenyl)-N-(cyclohexylmethyl)-4-methyl-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21250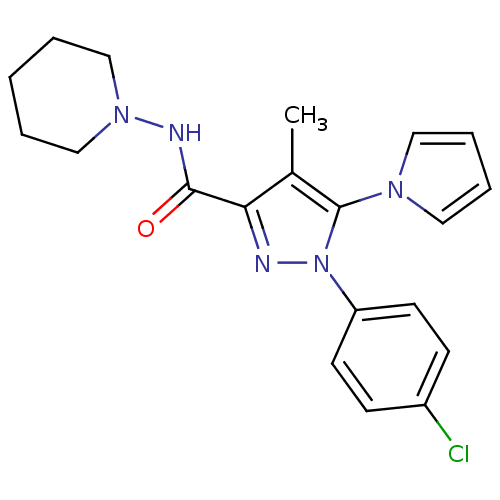 (1-(4-chlorophenyl)-4-methyl-N-(piperidin-1-yl)-5-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21276 (5-(2,5-dimethyl-1H-pyrrol-1-yl)-4-methyl-N-(piperi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21279 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21262 (1-(3,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21247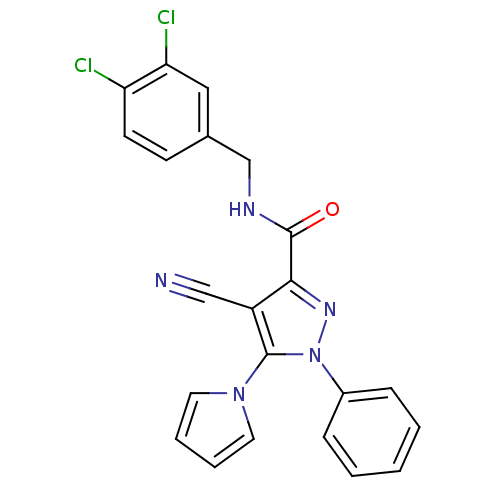 (4-cyano-N-[(3,4-dichlorophenyl)methyl]-1-phenyl-5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21275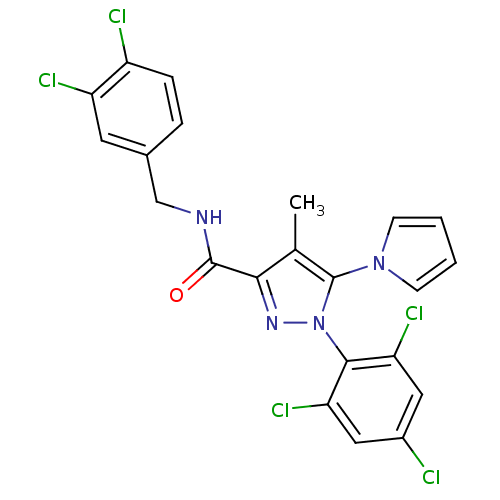 (N-[(3,4-dichlorophenyl)methyl]-4-methyl-5-(1H-pyrr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21249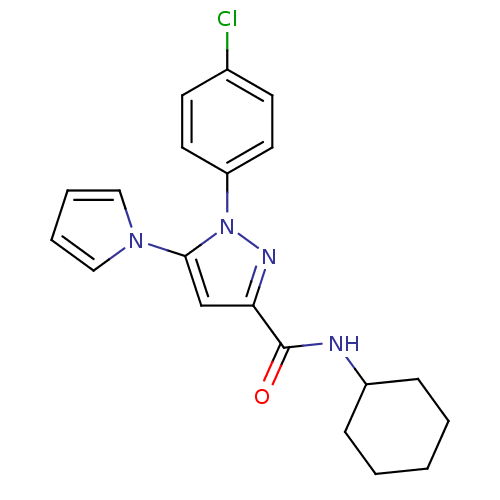 (1-(4-chlorophenyl)-N-cyclohexyl-5-(1H-pyrrol-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21265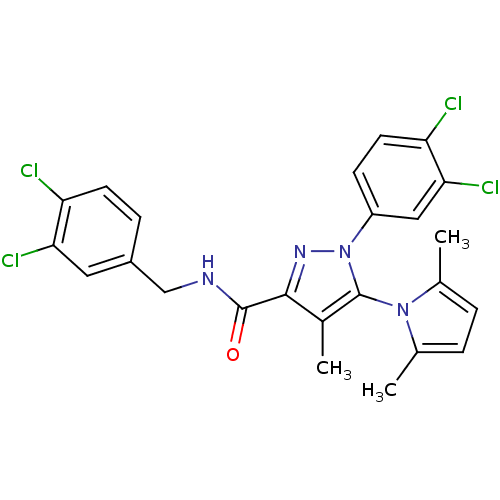 (1-(3,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21269 (N-[(3,4-dichlorophenyl)methyl]-1-(2,4-difluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21251 (1-(4-chlorophenyl)-N-(cyclohexylmethyl)-4-methyl-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21277 (N-cyclohexyl-5-(2,5-dimethyl-1H-pyrrol-1-yl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21256 (1-(2,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21273 (N-cyclohexyl-1-(2,4-difluorophenyl)-5-(2,5-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21258 (1-(2,4-dichlorophenyl)-5-(2,5-dimethyl-1H-pyrrol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21265 (1-(3,4-dichlorophenyl)-N-[(3,4-dichlorophenyl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21270 (N-cyclohexyl-1-(2,4-difluorophenyl)-4-methyl-5-(1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21271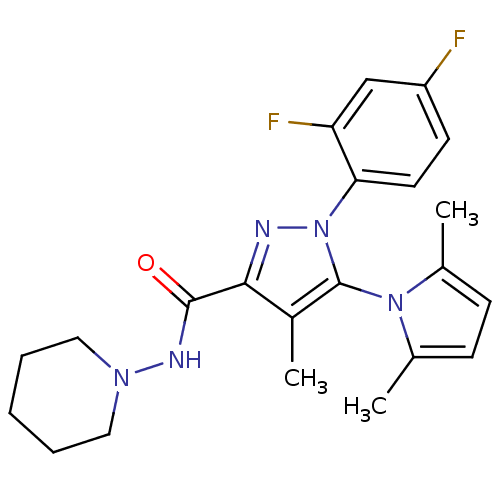 (1-(2,4-difluorophenyl)-5-(2,5-dimethyl-1H-pyrrol-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21255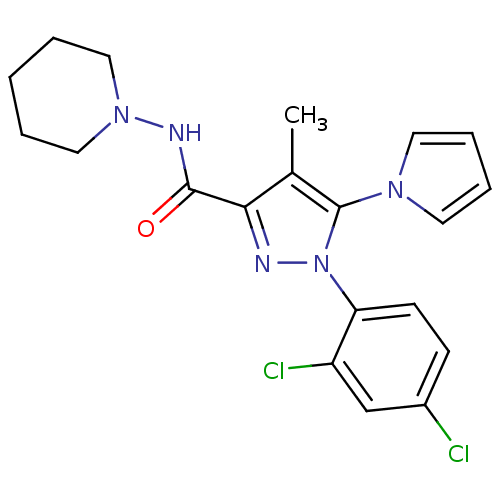 (1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21249 (1-(4-chlorophenyl)-N-cyclohexyl-5-(1H-pyrrol-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21274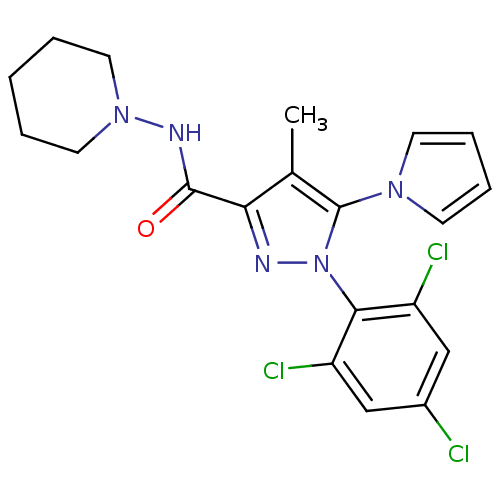 (4-methyl-N-(piperidin-1-yl)-5-(1H-pyrrol-1-yl)-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21255 (1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21250 (1-(4-chlorophenyl)-4-methyl-N-(piperidin-1-yl)-5-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21267 (1-(2,4-difluorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21267 (1-(2,4-difluorophenyl)-4-methyl-N-(piperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21269 (N-[(3,4-dichlorophenyl)methyl]-1-(2,4-difluorophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Sapienza Universitāadi Roma | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1560-76 (2008) Article DOI: 10.1021/jm070566z BindingDB Entry DOI: 10.7270/Q2GX48V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |