

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21351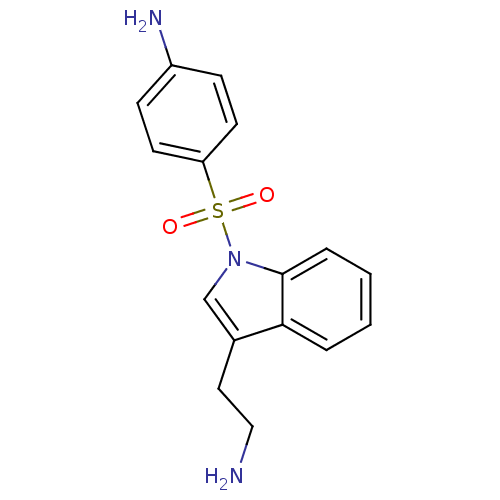 (4-{[3-(2-aminoethyl)-1H-indole-1-]sulfonyl}aniline...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | 91 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.2 | n/a | n/a | 6.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21345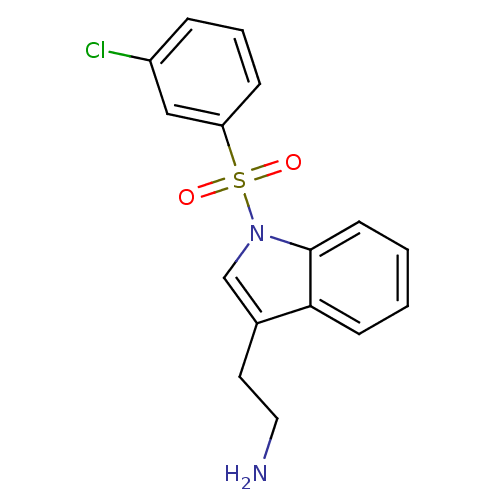 (2-{1-[(3-chlorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 109 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21354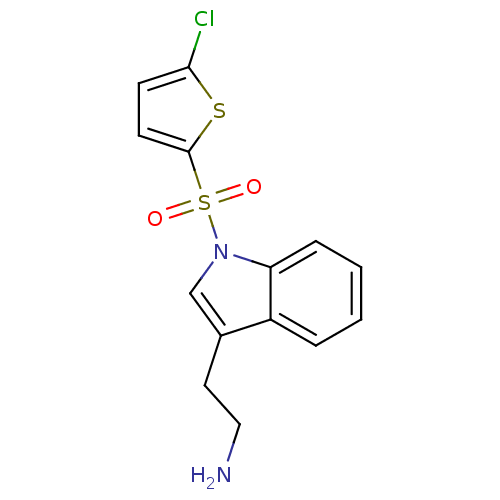 (2-{1-[(5-chlorothiophene-2-)sulfonyl]-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 181 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21355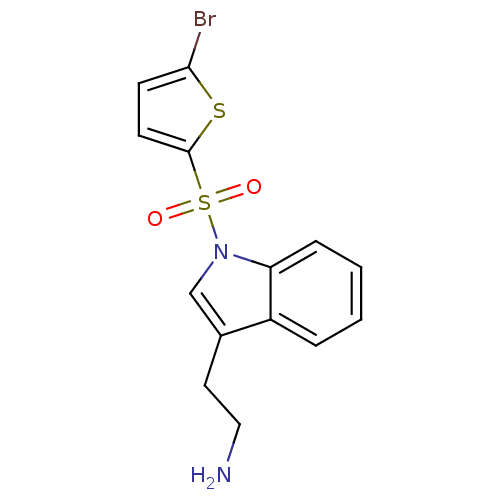 (2-{1-[(5-bromothiophene-2-)sulfonyl]-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.1 | n/a | n/a | 54 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21353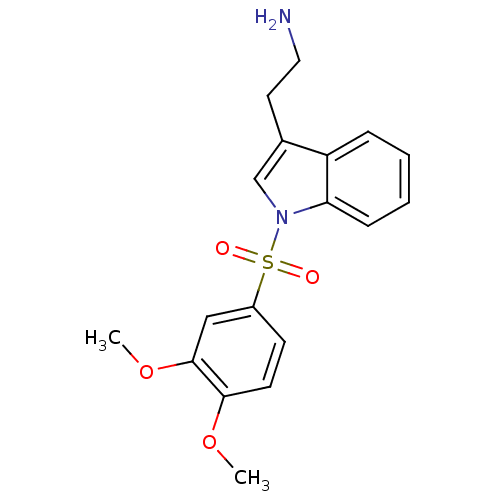 (2-{1-[(3,4-dimethoxybenzene)sulfonyl]-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.1 | n/a | n/a | 17.5 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21365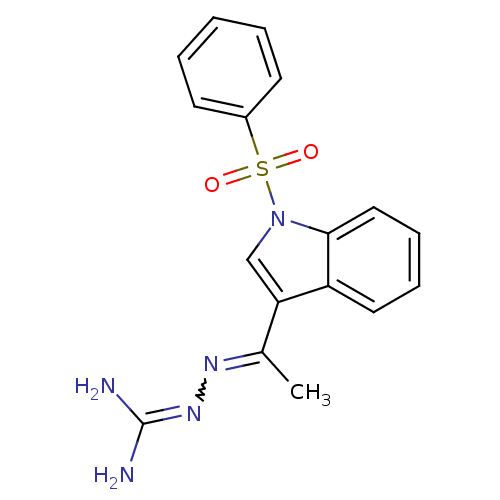 (3-[(E)-{1-[1-(benzenesulfonyl)-1H-indol-3-yl]ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21349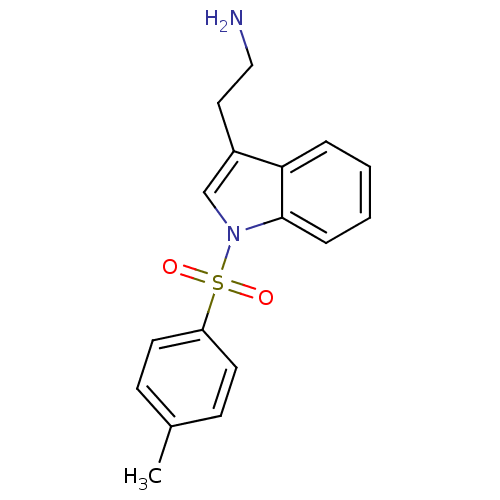 (2-{1-[(4-methylbenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | 191 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21357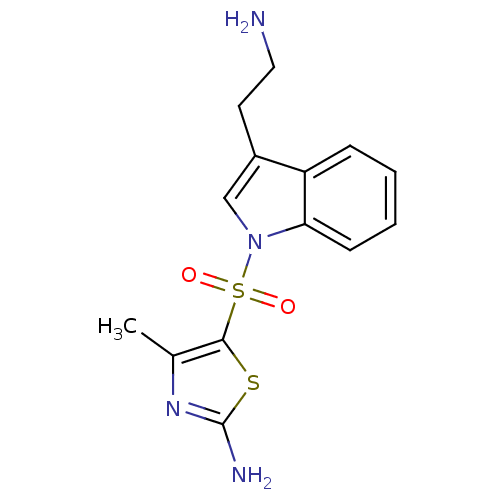 (5-thiazolylsulfonyl tryptamine, 11p | 5-{[3-(2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 9.60 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21341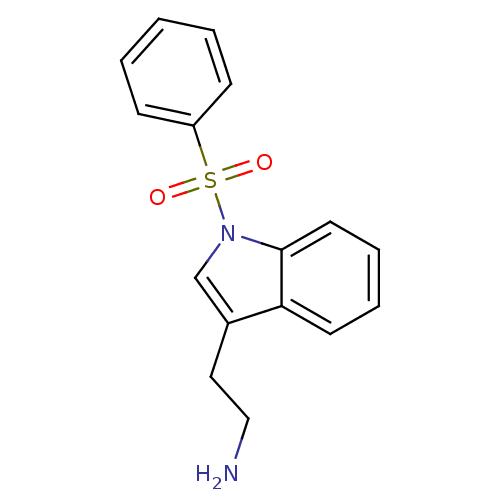 (2-[1-(benzenesulfonyl)-1H-indol-3-yl]ethan-1-amine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 73 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21347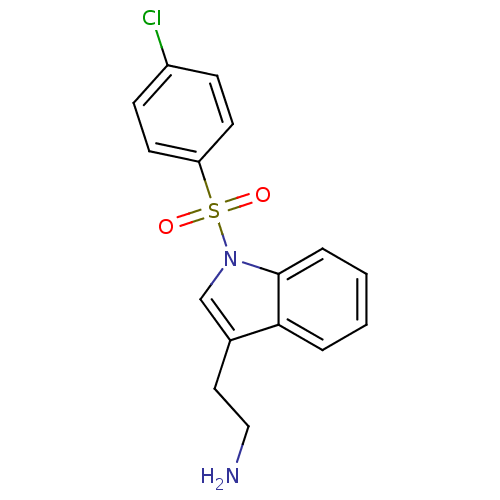 (2-{1-[(4-chlorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 174 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21366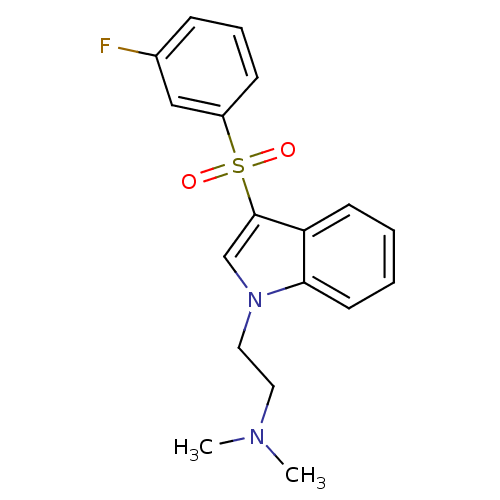 ((2-{3-[(3-fluorobenzene)sulfonyl]-1H-indol-1-yl}et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21343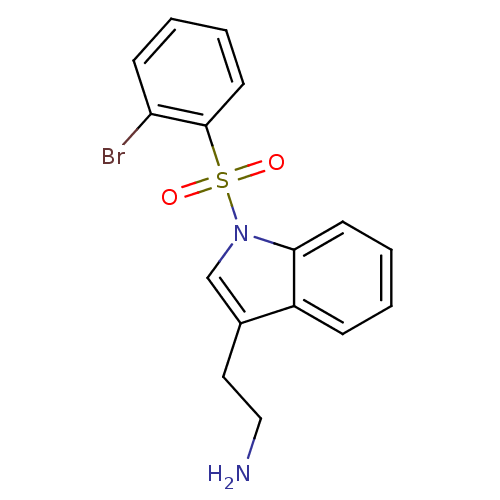 (2-{1-[(2-bromobenzene)sulfonyl]-1H-indol-3-yl}etha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -45.0 | n/a | n/a | 148 | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21348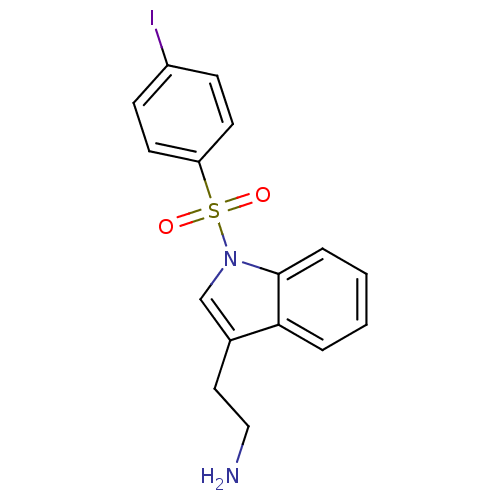 (2-{1-[(4-iodobenzene)sulfonyl]-1H-indol-3-yl}ethan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21344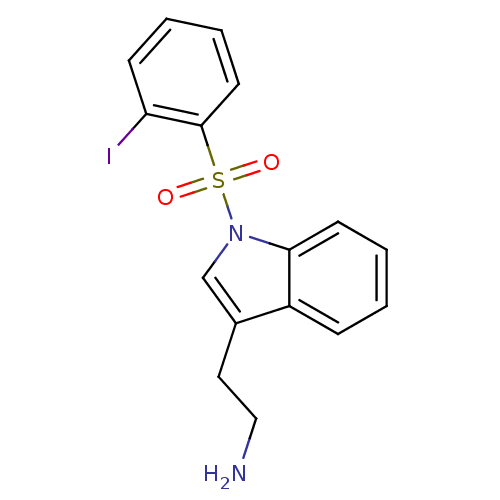 (2-{1-[(2-iodobenzene)sulfonyl]-1H-indol-3-yl}ethan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21346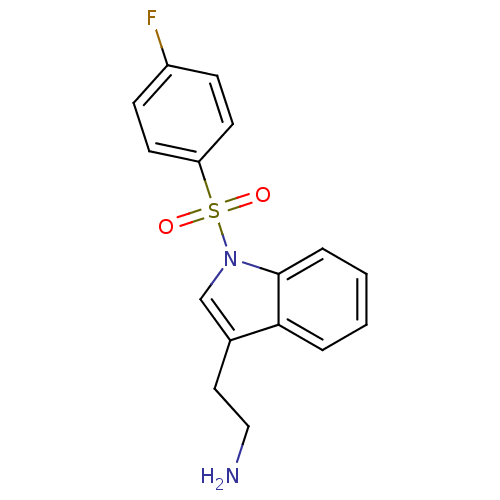 (2-{1-[(4-fluorobenzene)sulfonyl]-1H-indol-3-yl}eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21364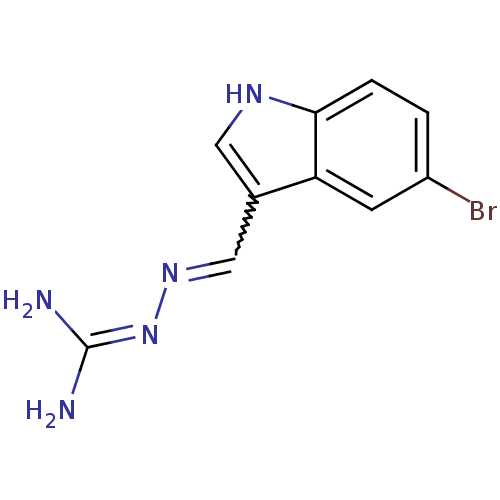 (3-[(E)-[(5-bromo-1H-indol-3-yl)methylidene]amino]g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21352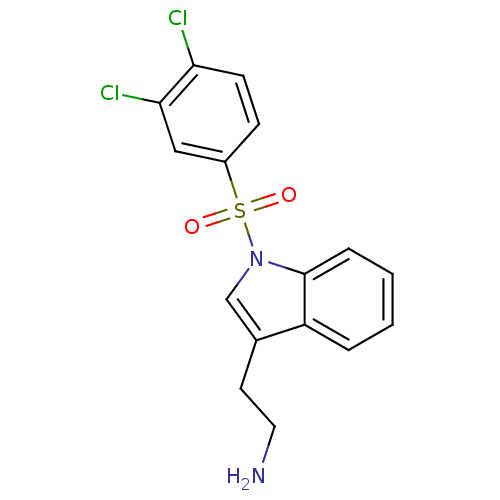 (2-{1-[(3,4-dichlorobenzene)sulfonyl]-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21356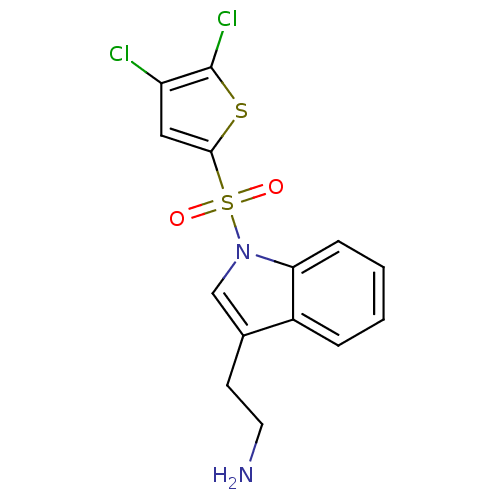 (2-{1-[(4,5-dichlorothiophene-2-)sulfonyl]-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21350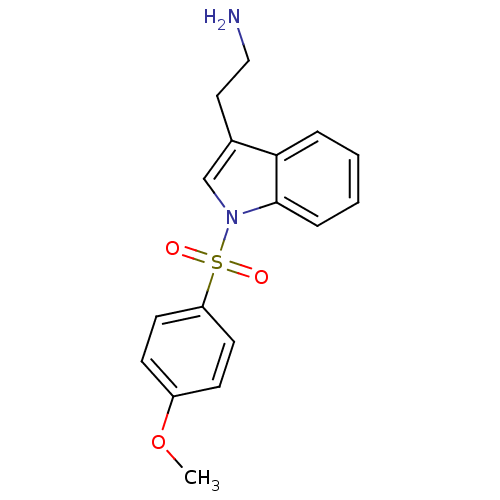 (2-{1-[(4-methoxybenzene)sulfonyl]-1H-indol-3-yl}et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 458 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 679 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||