

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398095 (CHEMBL2182043 | US9321738, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398093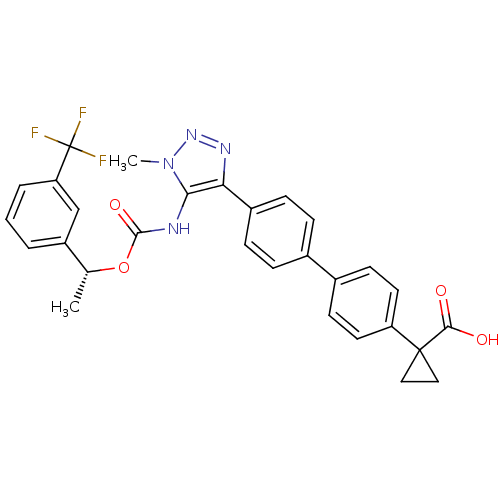 (CHEMBL2182046 | US9321738, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398094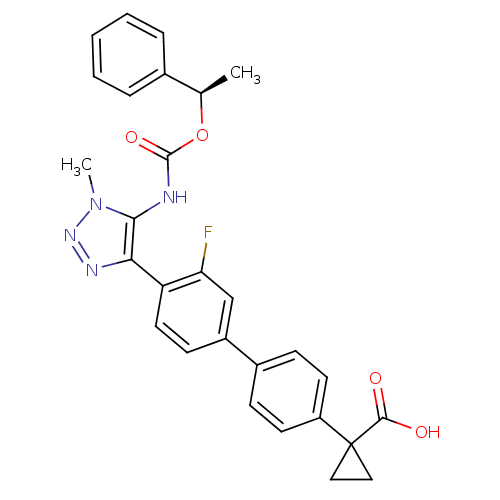 (CHEMBL2182044 | US9321738, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398091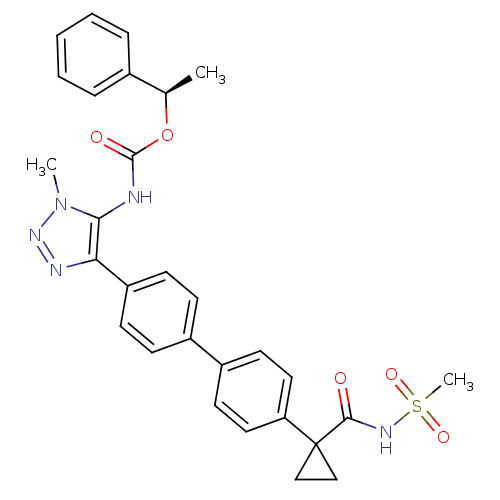 (CHEMBL2182049 | US9321738, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398090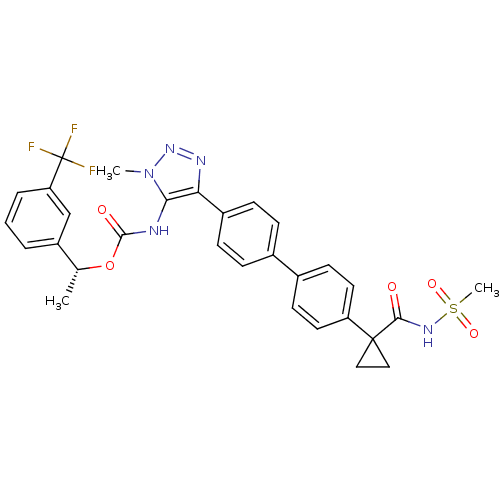 (CHEMBL2182050 | US9321738, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM223530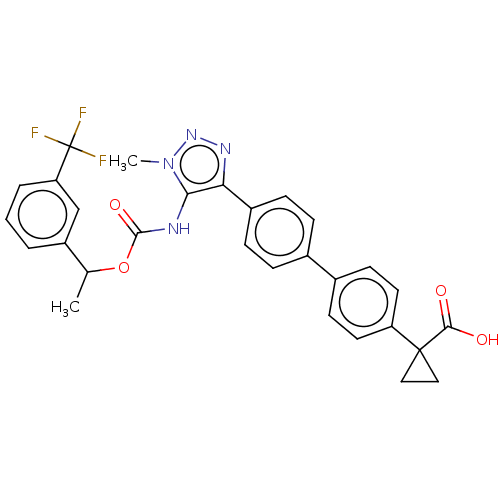 (US9321738, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398096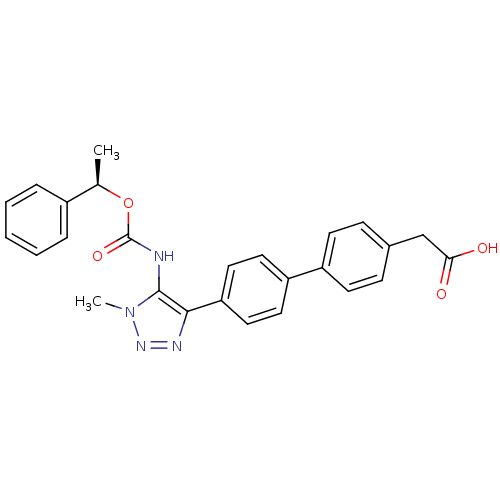 (CHEMBL2182042 | US9321738, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398089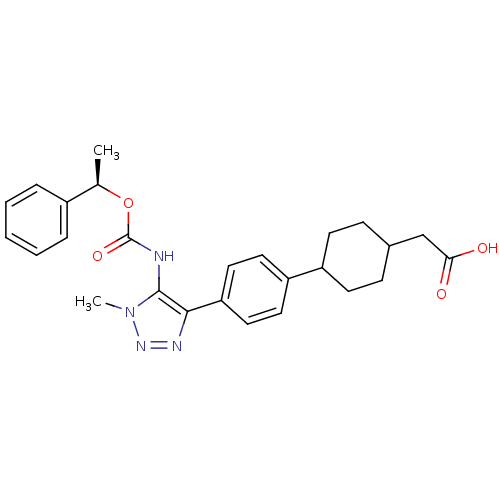 (CHEMBL2182051 | US9321738, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398090 (CHEMBL2182050 | US9321738, 13) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65.7 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398112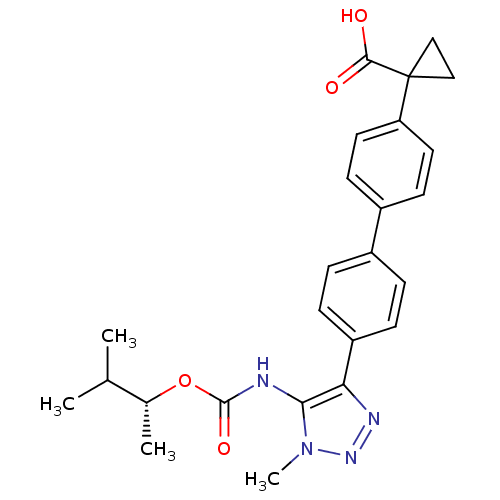 (CHEMBL2182045 | US9321738, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398093 (CHEMBL2182046 | US9321738, 6) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM223530 (US9321738, 4) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398095 (CHEMBL2182043 | US9321738, 2) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398091 (CHEMBL2182049 | US9321738, 12) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398092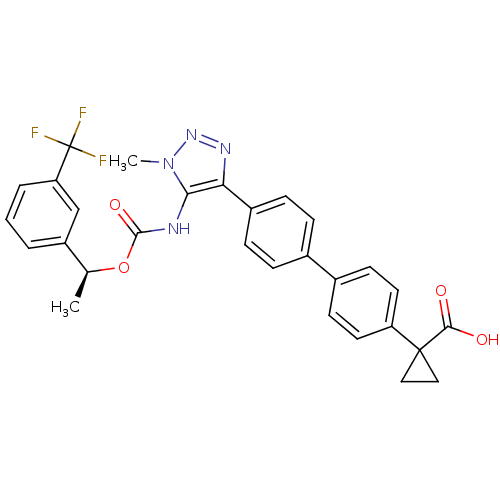 (CHEMBL2182047 | US9321738, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM223532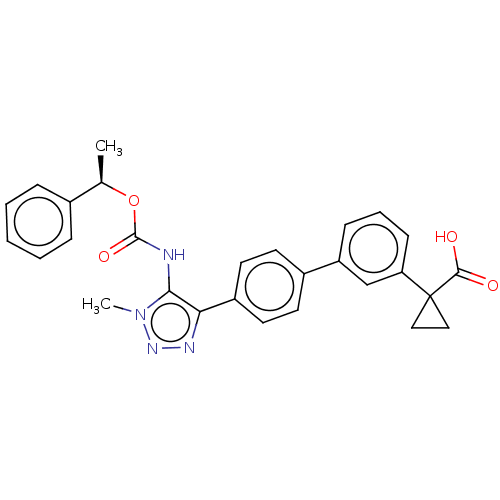 (US9321738, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398092 (CHEMBL2182047 | US9321738, 5) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM223532 (US9321738, 9) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398089 (CHEMBL2182051 | US9321738, 14) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM223529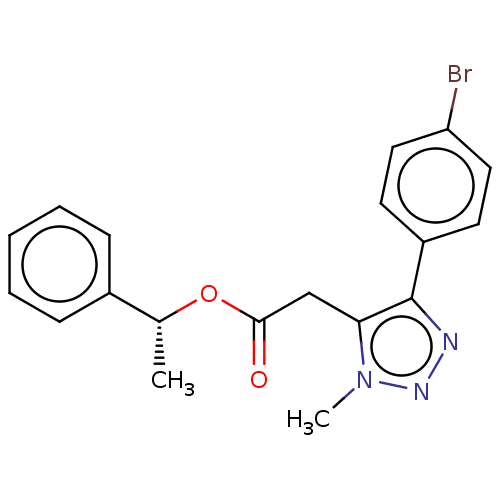 (US9321738, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398096 (CHEMBL2182042 | US9321738, 3) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398094 (CHEMBL2182044 | US9321738, 11) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM223531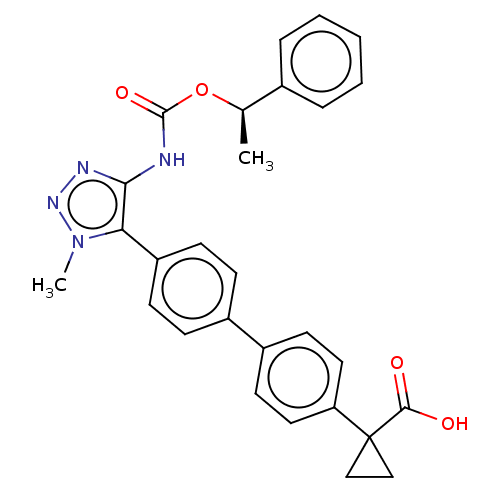 (US9321738, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM223529 (US9321738, 1) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM223531 (US9321738, 8) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398112 (CHEMBL2182045 | US9321738, 7) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM223533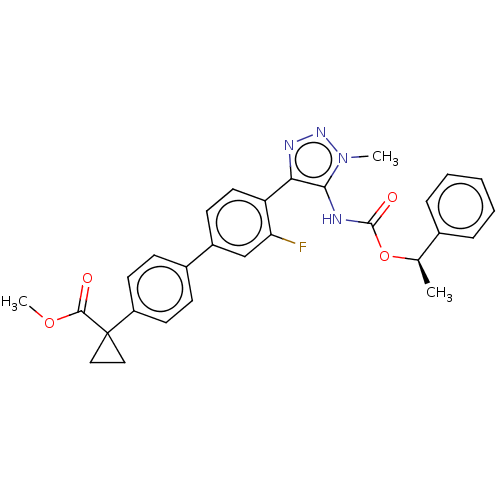 (US9321738, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM223533 (US9321738, 10) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||