

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM22113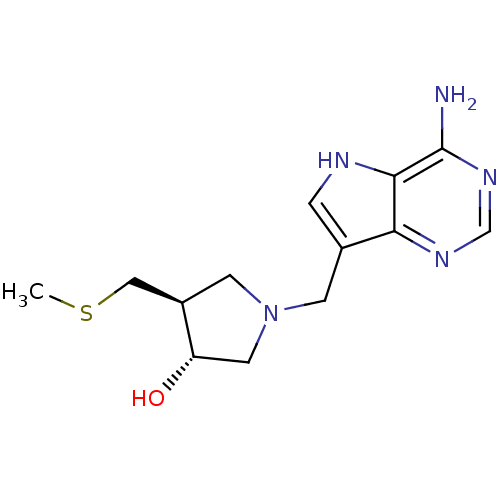 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0480 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22103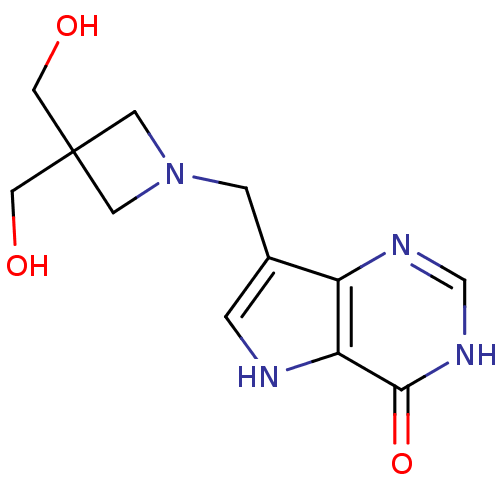 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.229 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM22112 (7-({3-[(methylsulfanyl)methyl]azetidin-1-yl}methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | -52.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22109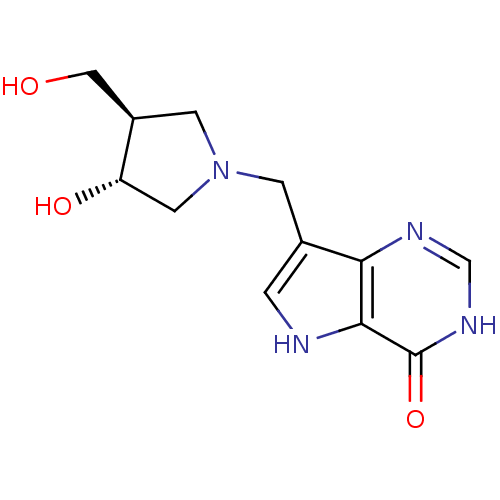 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22103 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.665 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM22110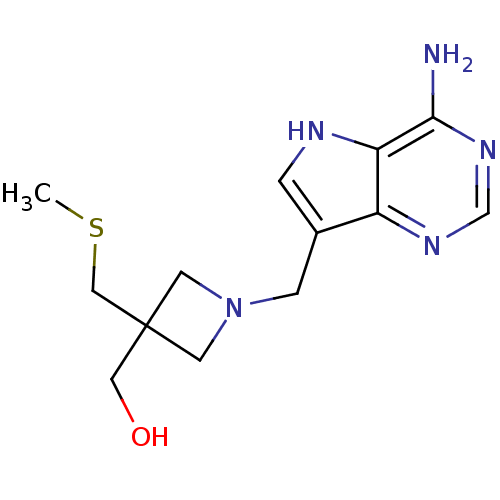 (Azetidine based compound, 43 | [1-({4-amino-5H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22108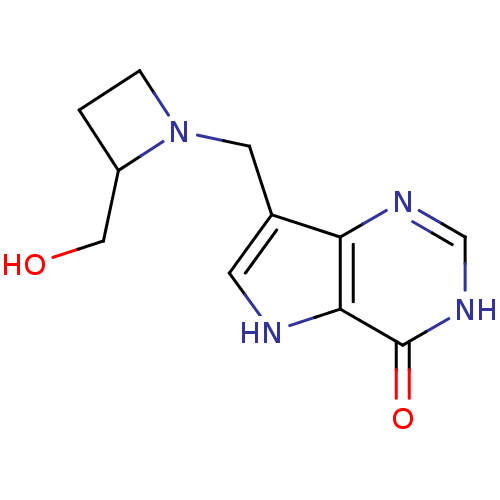 (7-{[2-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22108 (7-{[2-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22112 (7-({3-[(methylsulfanyl)methyl]azetidin-1-yl}methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22105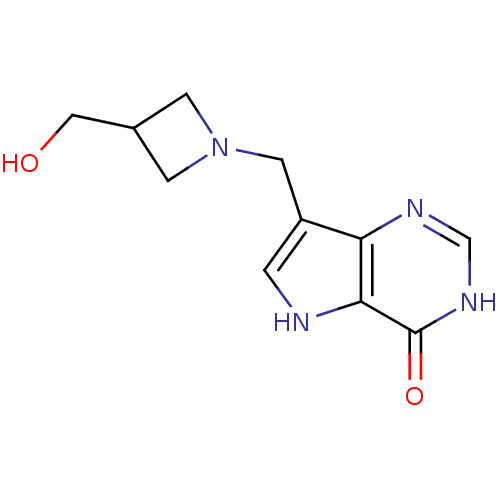 (7-{[3-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22105 (7-{[3-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22106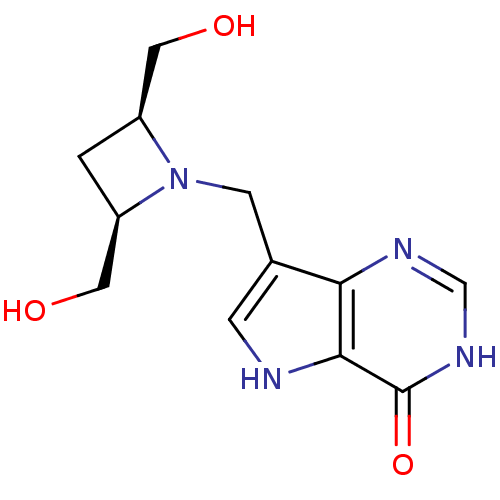 (7-{[(2R,4S)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12.9 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22106 (7-{[(2R,4S)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteine nucleosidase (Streptococcus pneumoniae) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteine nucleosidase (Streptococcus pneumoniae) | BDBM22112 (7-({3-[(methylsulfanyl)methyl]azetidin-1-yl}methyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 84 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22110 (Azetidine based compound, 43 | [1-({4-amino-5H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteine nucleosidase (Streptococcus pneumoniae) | BDBM22110 (Azetidine based compound, 43 | [1-({4-amino-5H-pyr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22108 (7-{[2-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 191 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22107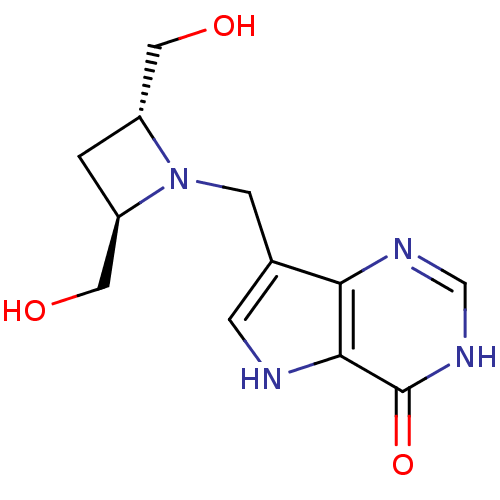 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM22107 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -36.4 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22107 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | -35.2 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22106 (7-{[(2R,4S)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22105 (7-{[3-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.3 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22103 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.3 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||