 Found 64 hits Enz. Inhib. hit(s) with all data for entry = 8236
Found 64 hits Enz. Inhib. hit(s) with all data for entry = 8236 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26190

(1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...)Show InChI InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237299
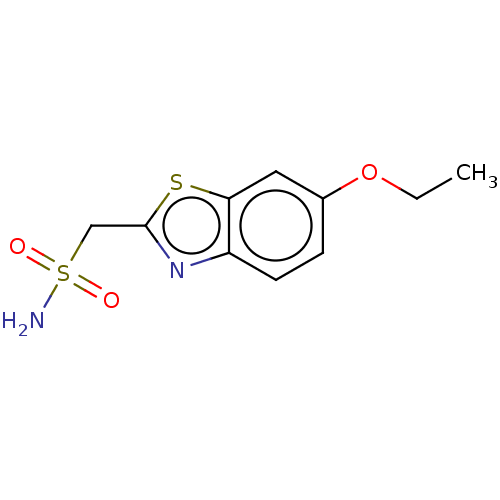
(Ethoxzolamide (EZA))Show InChI InChI=1S/C10H12N2O3S2/c1-2-15-7-3-4-8-9(5-7)16-10(12-8)6-17(11,13)14/h3-5H,2,6H2,1H3,(H2,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50031472
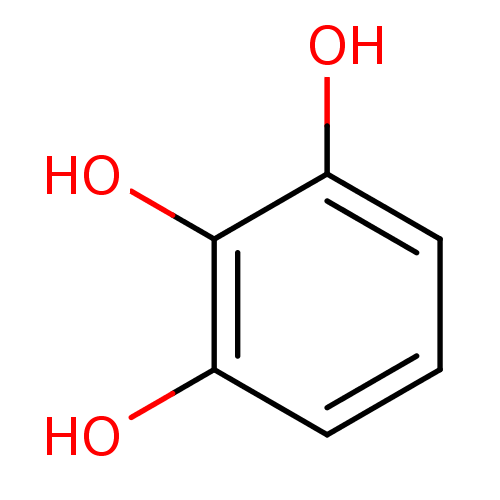
(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237299

(Ethoxzolamide (EZA))Show InChI InChI=1S/C10H12N2O3S2/c1-2-15-7-3-4-8-9(5-7)16-10(12-8)6-17(11,13)14/h3-5H,2,6H2,1H3,(H2,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10888
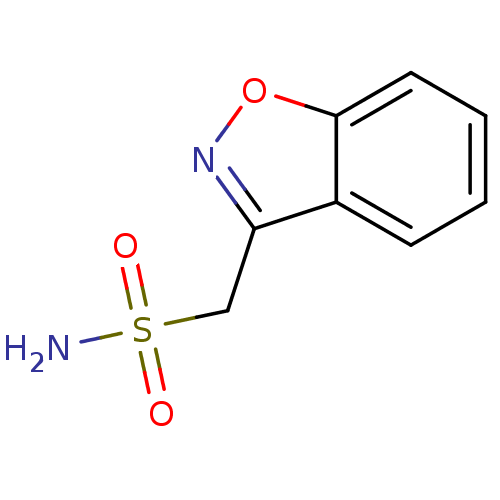
(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237297
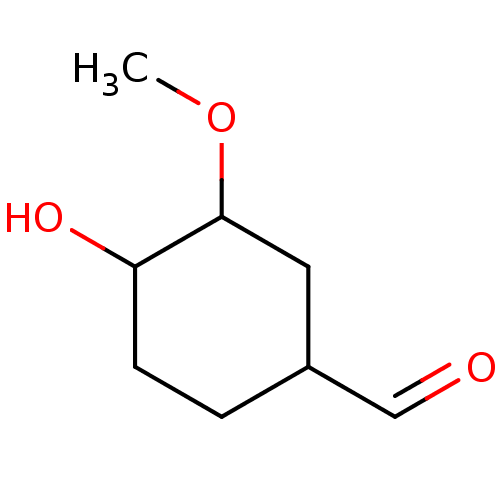
(Vanillin (14))Show InChI InChI=1S/C8H14O3/c1-11-8-4-6(5-9)2-3-7(8)10/h5-8,10H,2-4H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM237299

(Ethoxzolamide (EZA))Show InChI InChI=1S/C10H12N2O3S2/c1-2-15-7-3-4-8-9(5-7)16-10(12-8)6-17(11,13)14/h3-5H,2,6H2,1H3,(H2,11,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237297

(Vanillin (14))Show InChI InChI=1S/C8H14O3/c1-11-8-4-6(5-9)2-3-7(8)10/h5-8,10H,2-4H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM10888

(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240369
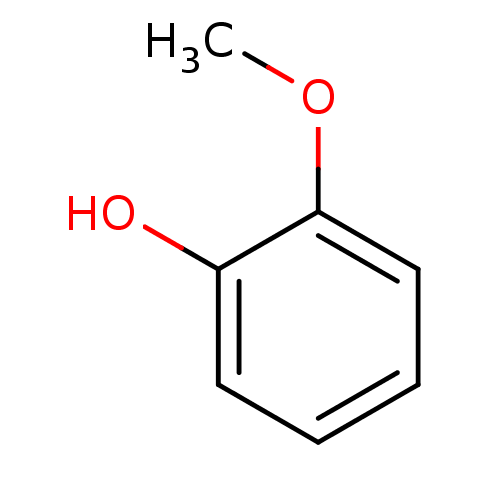
(1-Hydroxy-2-methoxybenzene | 2-Hydroxyanisole | 2-...)Show InChI InChI=1S/C7H8O2/c1-9-7-5-3-2-4-6(7)8/h2-5,8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM237297

(Vanillin (14))Show InChI InChI=1S/C8H14O3/c1-11-8-4-6(5-9)2-3-7(8)10/h5-8,10H,2-4H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50240369

(1-Hydroxy-2-methoxybenzene | 2-Hydroxyanisole | 2-...)Show InChI InChI=1S/C7H8O2/c1-9-7-5-3-2-4-6(7)8/h2-5,8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM237299

(Ethoxzolamide (EZA))Show InChI InChI=1S/C10H12N2O3S2/c1-2-15-7-3-4-8-9(5-7)16-10(12-8)6-17(11,13)14/h3-5H,2,6H2,1H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237294
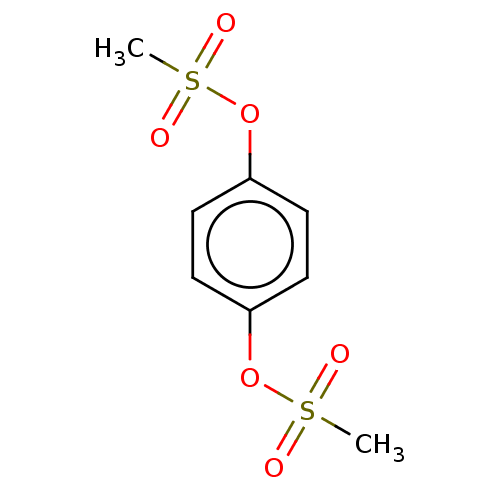
(1,4-Phenylene dimethanesulfonate (6))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-3-5-8(6-4-7)14-16(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26187
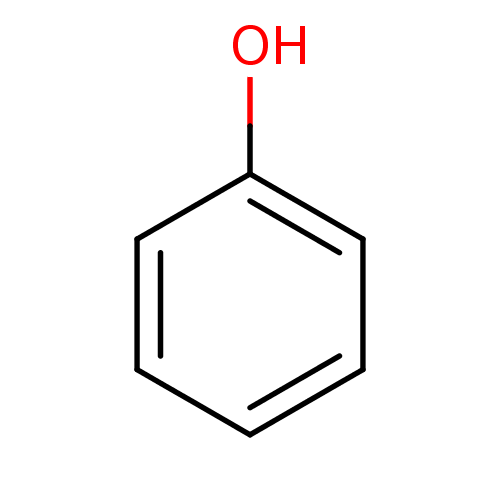
(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237298
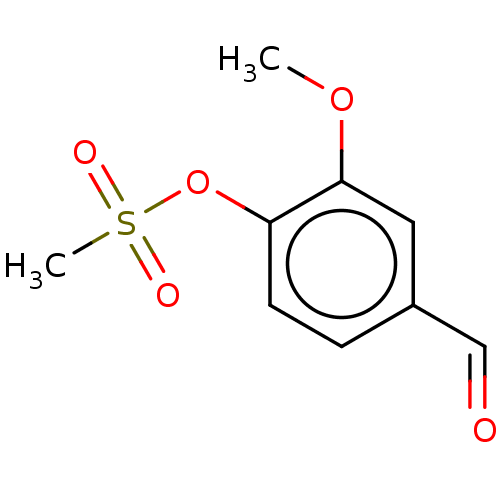
(4-Formyl-2-methoxyphenyl methanesulfonate (15))Show InChI InChI=1S/C9H10O5S/c1-13-9-5-7(6-10)3-4-8(9)14-15(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50149238
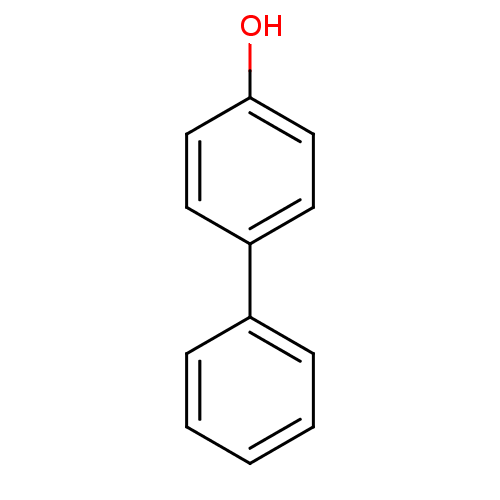
(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237298

(4-Formyl-2-methoxyphenyl methanesulfonate (15))Show InChI InChI=1S/C9H10O5S/c1-13-9-5-7(6-10)3-4-8(9)14-15(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237292
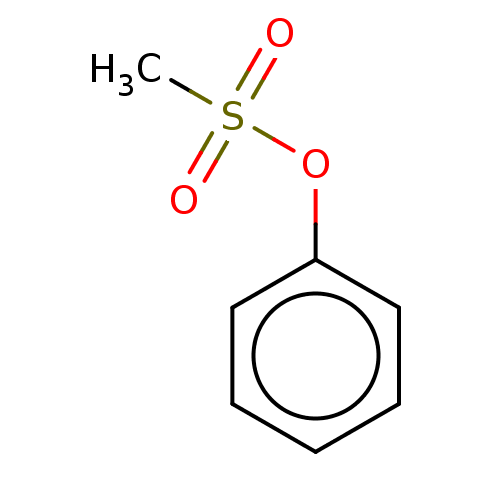
(Phenylmethanesulfonate (4))Show InChI InChI=1S/C7H8O3S/c1-11(8,9)10-7-5-3-2-4-6-7/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237294

(1,4-Phenylene dimethanesulfonate (6))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-3-5-8(6-4-7)14-16(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM26187

(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26188
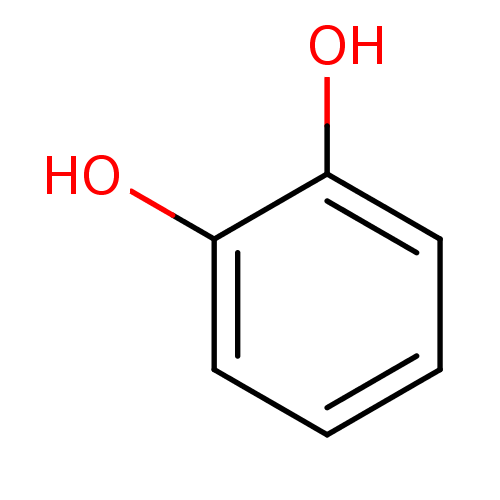
(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26187

(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26190

(1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...)Show InChI InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM26190

(1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...)Show InChI InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM26188

(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM237298

(4-Formyl-2-methoxyphenyl methanesulfonate (15))Show InChI InChI=1S/C9H10O5S/c1-13-9-5-7(6-10)3-4-8(9)14-15(2,11)12/h3-6H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237293

(Biphenyl-4-ylmethanesulfonate (5))Show InChI InChI=1S/C13H12O3S/c1-17(14,15)16-13-9-7-12(8-10-13)11-5-3-2-4-6-11/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50149238

(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10888

(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237292

(Phenylmethanesulfonate (4))Show InChI InChI=1S/C7H8O3S/c1-11(8,9)10-7-5-3-2-4-6-7/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50149238

(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM237294

(1,4-Phenylene dimethanesulfonate (6))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-3-5-8(6-4-7)14-16(2,11)12/h3-6H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237293

(Biphenyl-4-ylmethanesulfonate (5))Show InChI InChI=1S/C13H12O3S/c1-17(14,15)16-13-9-7-12(8-10-13)11-5-3-2-4-6-11/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM237292

(Phenylmethanesulfonate (4))Show InChI InChI=1S/C7H8O3S/c1-11(8,9)10-7-5-3-2-4-6-7/h2-6H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM237294

(1,4-Phenylene dimethanesulfonate (6))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-3-5-8(6-4-7)14-16(2,11)12/h3-6H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237296
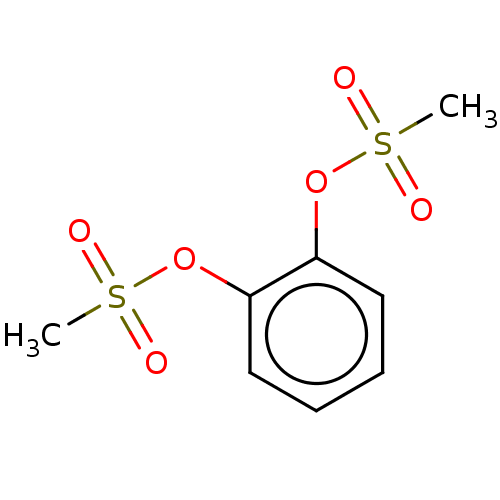
(1,2-Phenylenedimethanesulfonate (13))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-5-3-4-6-8(7)14-16(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM237295
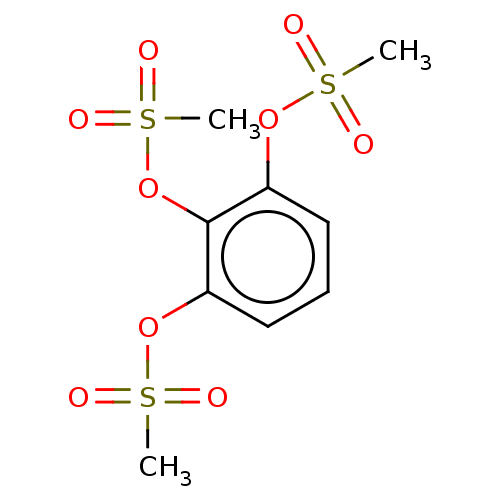
(Benzene-1,2,3-triyltrimethanesulfonate (11))Show SMILES CS(=O)(=O)Oc1cccc(OS(C)(=O)=O)c1OS(C)(=O)=O Show InChI InChI=1S/C9H12O9S3/c1-19(10,11)16-7-5-4-6-8(17-20(2,12)13)9(7)18-21(3,14)15/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10888

(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237296

(1,2-Phenylenedimethanesulfonate (13))Show InChI InChI=1S/C8H10O6S2/c1-15(9,10)13-7-5-3-4-6-8(7)14-16(2,11)12/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM237295

(Benzene-1,2,3-triyltrimethanesulfonate (11))Show SMILES CS(=O)(=O)Oc1cccc(OS(C)(=O)=O)c1OS(C)(=O)=O Show InChI InChI=1S/C9H12O9S3/c1-19(10,11)16-7-5-4-6-8(17-20(2,12)13)9(7)18-21(3,14)15/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM237293

(Biphenyl-4-ylmethanesulfonate (5))Show InChI InChI=1S/C13H12O3S/c1-17(14,15)16-13-9-7-12(8-10-13)11-5-3-2-4-6-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM237297

(Vanillin (14))Show InChI InChI=1S/C8H14O3/c1-11-8-4-6(5-9)2-3-7(8)10/h5-8,10H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data