

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238307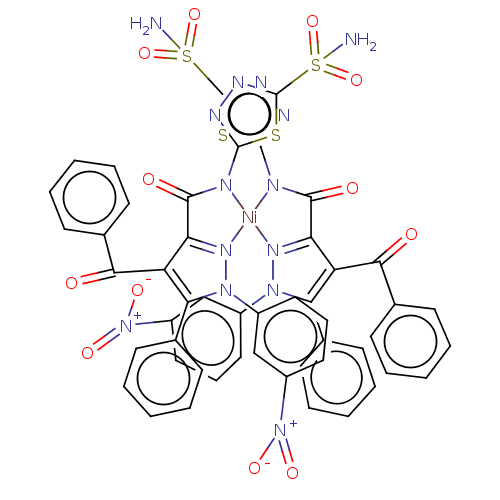 ([NiL2(H2O)2] (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238307 ([NiL2(H2O)2] (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238308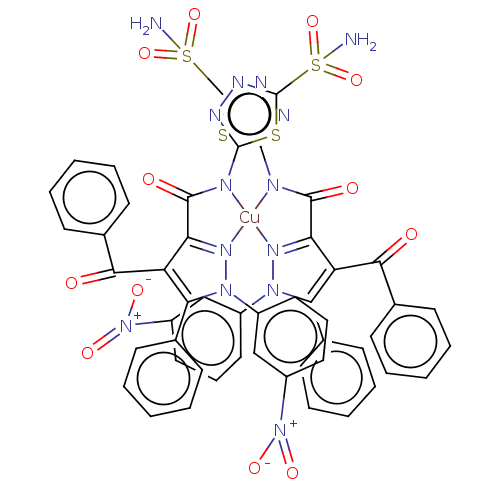 ([CuL2(H2O)2].H2O (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238308 ([CuL2(H2O)2].H2O (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238305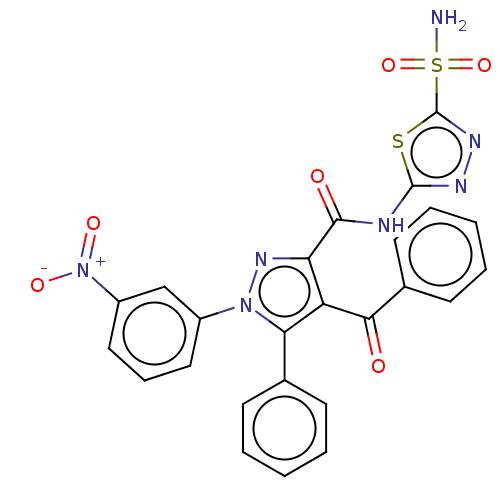 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238305 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The method for determination of Ki values is described elsewhere [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzy... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238307 ([NiL2(H2O)2] (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238307 ([NiL2(H2O)2] (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238307 ([NiL2(H2O)2] (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238307 ([NiL2(H2O)2] (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238308 ([CuL2(H2O)2].H2O (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238308 ([CuL2(H2O)2].H2O (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238308 ([CuL2(H2O)2].H2O (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238305 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238306 ([CoL2(H2O)2].2H2O (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238305 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM238305 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238308 ([CuL2(H2O)2].H2O (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM238305 (N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson [Wilbur et al., J.... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 311-5 (2013) Article DOI: 10.3109/14756366.2012.712516 BindingDB Entry DOI: 10.7270/Q2QN65NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||