

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23698 (4-chloro-1-[(4-fluorophenyl)carbonyl]-1H-pyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23700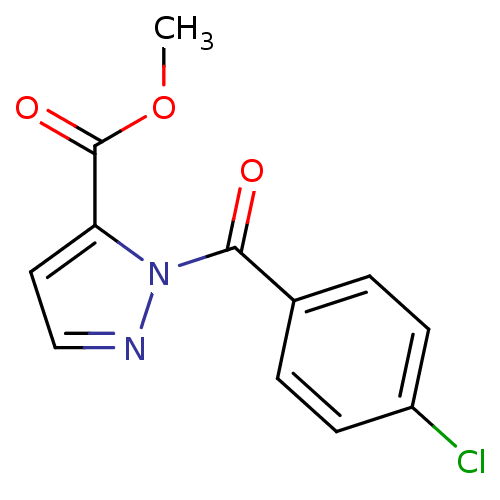 (N-Benzoylpyrazole deriv., 2 | methyl 1-[(4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23709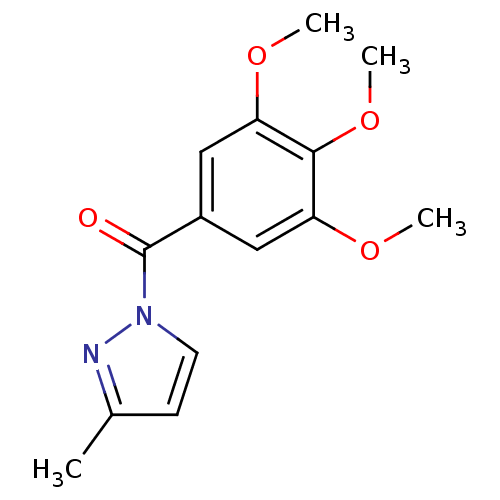 (3-methyl-1-[(3,4,5-trimethoxyphenyl)carbonyl]-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23701 (4-chloro-1-[(3-nitrophenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23702 (3-[(2,4-dimethylphenyl)carbonyl]-1-[(4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23710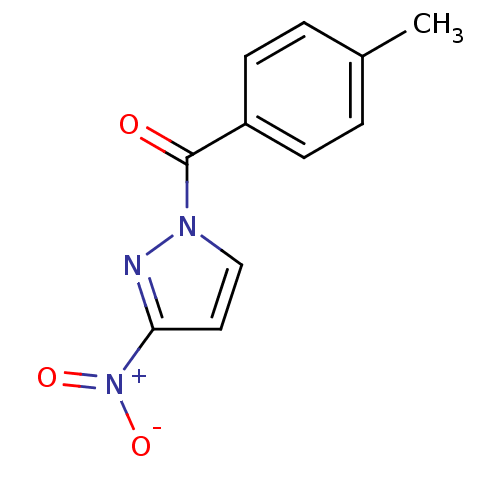 (1-[(4-methylphenyl)carbonyl]-3-nitro-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 28 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23703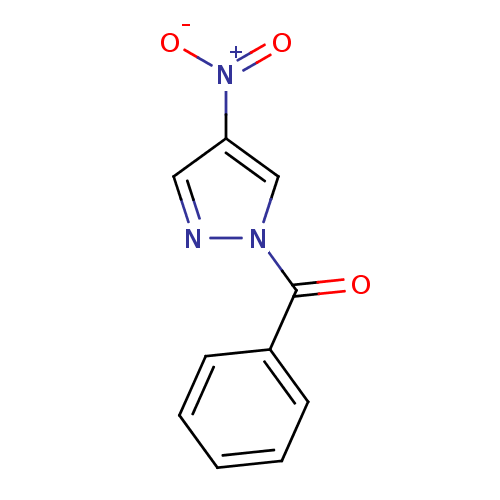 (1-benzoyl-4-nitro-1H-pyrazole | N-Benzoylpyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23704 (1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide | N-B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 39 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23705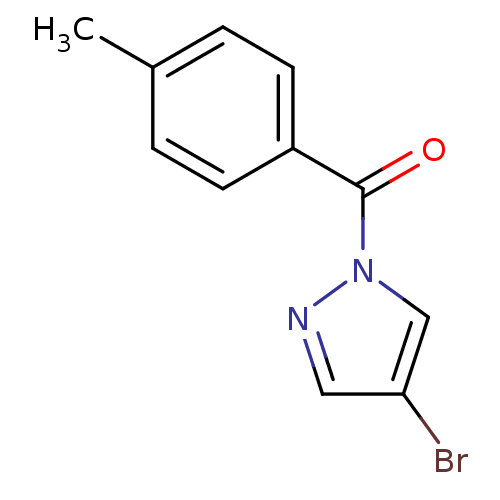 (4-bromo-1-[(4-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23711 (1-benzoyl-3-nitro-1H-pyrazole | N-Benzoylpyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 46 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23712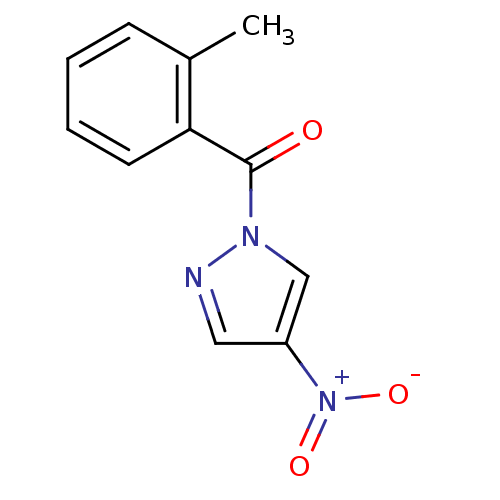 (1-[(2-methylphenyl)carbonyl]-4-nitro-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 65 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23706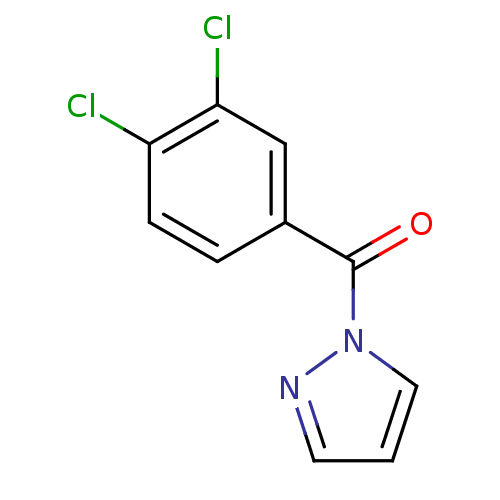 (1-[(3,4-dichlorophenyl)carbonyl]-1H-pyrazole | N-B...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 104 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23707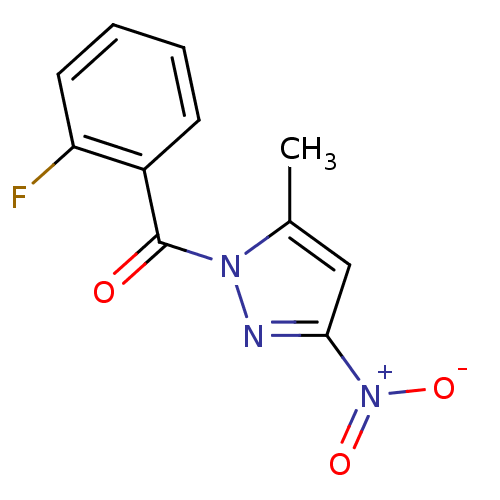 (1-[(2-fluorophenyl)carbonyl]-5-methyl-3-nitro-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 107 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23726 (1,1-dimethylprop-2-yn-1-yl 4-methoxybenzoate, 10 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23713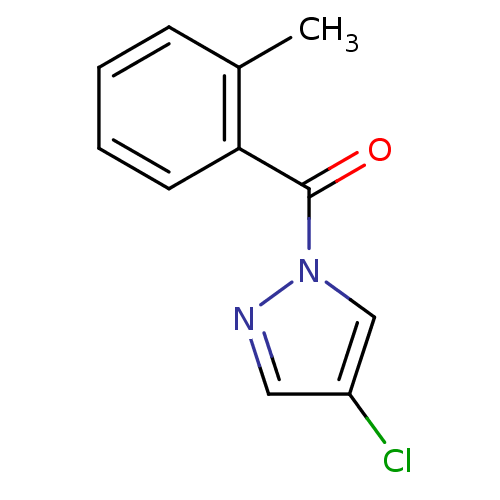 (4-chloro-1-[(2-methylphenyl)carbonyl]-1H-pyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 230 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23714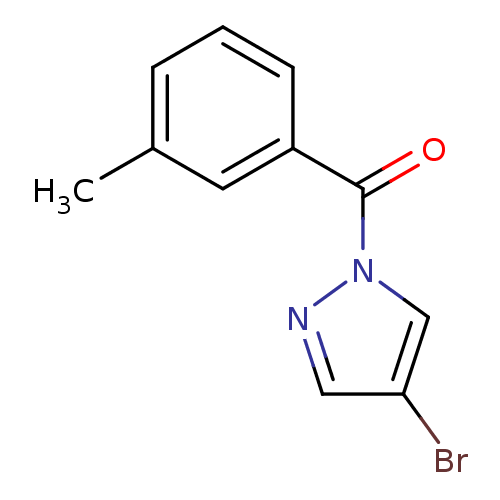 (4-bromo-1-[(3-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 250 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23727 (N,N-bis(cyanomethyl)-3,4-dimethoxybenzamide | N,N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 290 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23716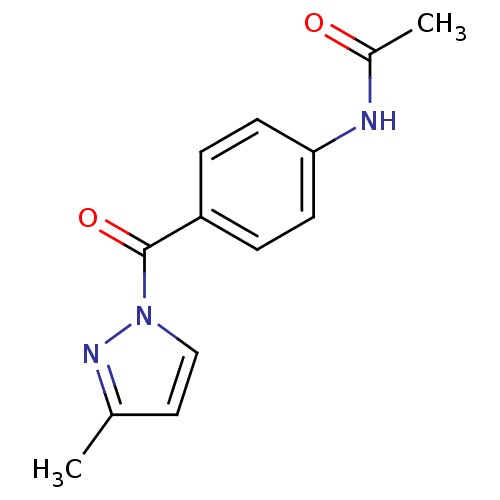 (N-Benzoylpyrazole deriv., 24 | N-{4-[(3-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23715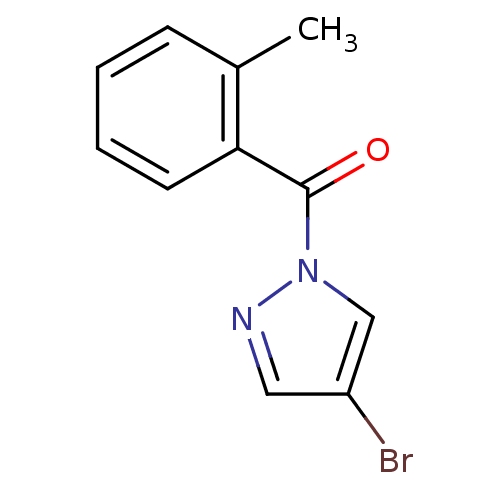 (4-bromo-1-[(2-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23728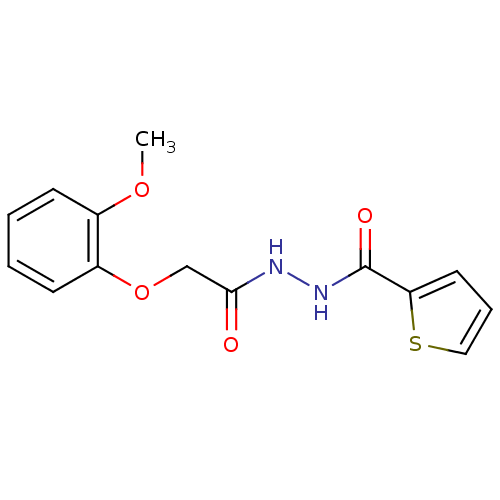 (2-(2-methoxyphenoxy)-N'-(thiophen-2-ylcarbonyl)ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 820 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23717 (4-chloro-1-[(2-chlorophenyl)carbonyl]-1H-pyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23708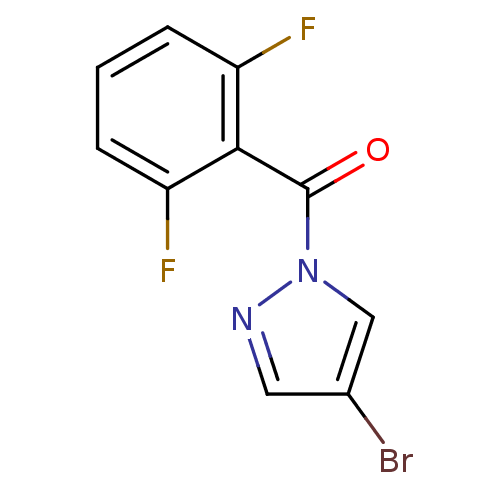 (4-bromo-1-[(2,6-difluorophenyl)carbonyl]-1H-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.10E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23730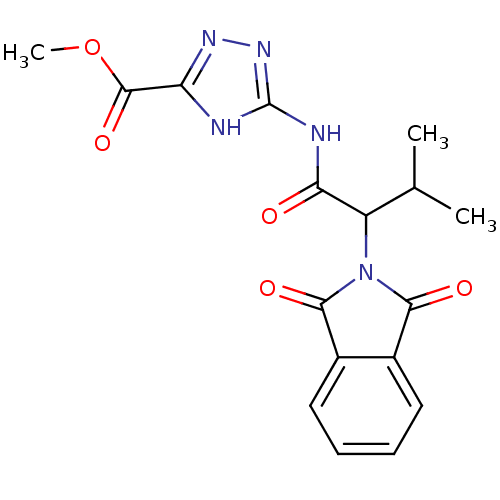 (1,2,4-triazole-5-carboxylate, 14 | methyl 3-[2-(1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23731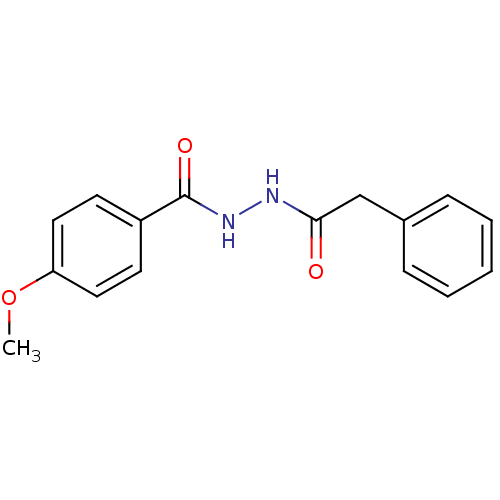 (4-methoxy-N -(phenylacetyl)benzohydrazide | 4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23732 (3-(2-chlorophenyl)-5-methyl-4-(1H-pyrazol-1-ylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23718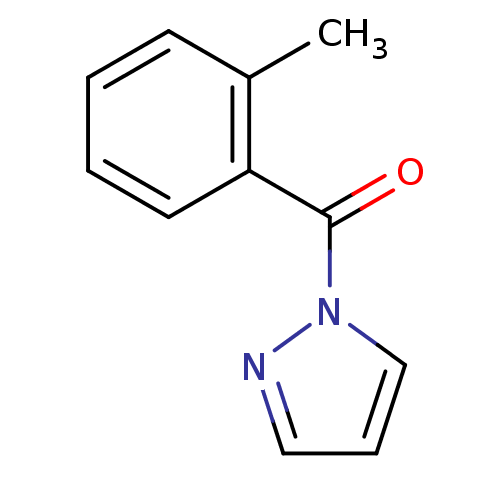 (1-[(2-methylphenyl)carbonyl]-1H-pyrazole | N-Benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23719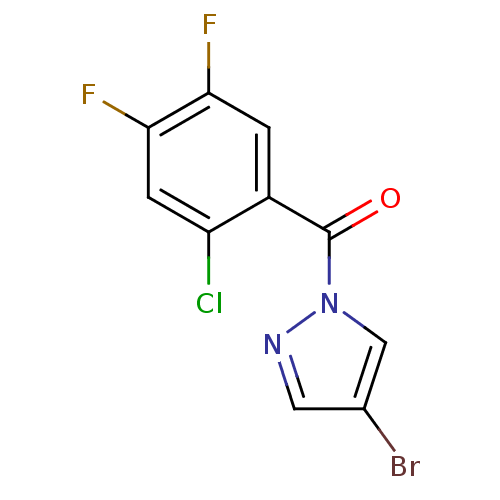 (4-bromo-1-[(2-chloro-4,5-difluorophenyl)carbonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.20E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23720 (1-[(4-tert-butylphenyl)carbonyl]-4-chloro-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23721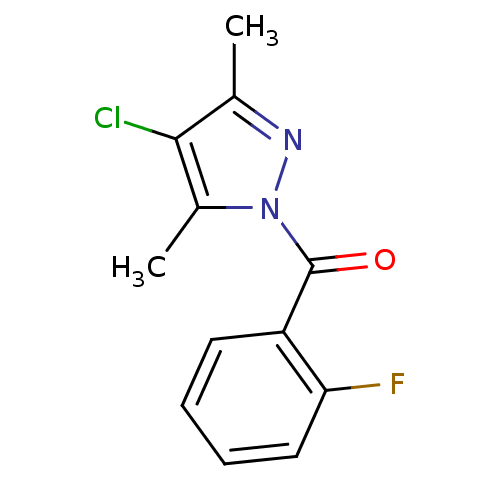 (4-chloro-1-[(2-fluorophenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23722 (4-chloro-1-[(4-chlorophenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23723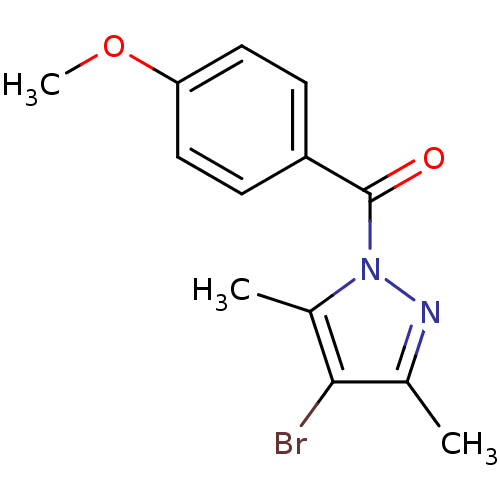 (4-bromo-1-[(4-methoxyphenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.45E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23724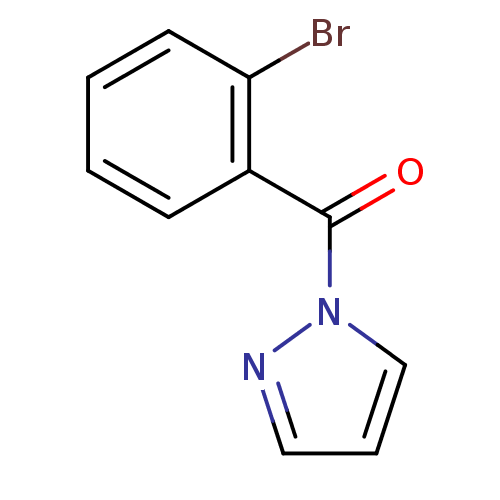 (1-[(2-bromophenyl)carbonyl]-1H-pyrazole | N-Benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.99E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23725 (3,4,5-trimethyl-1-[(3,4,5-trimethoxyphenyl)carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.09E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23705 (4-bromo-1-[(4-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23700 (N-Benzoylpyrazole deriv., 2 | methyl 1-[(4-chlorop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23698 (4-chloro-1-[(4-fluorophenyl)carbonyl]-1H-pyrazole ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23709 (3-methyl-1-[(3,4,5-trimethoxyphenyl)carbonyl]-1H-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23700 (N-Benzoylpyrazole deriv., 2 | methyl 1-[(4-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23704 (1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide | N-B...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23702 (3-[(2,4-dimethylphenyl)carbonyl]-1-[(4-fluoropheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23700 (N-Benzoylpyrazole deriv., 2 | methyl 1-[(4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23702 (3-[(2,4-dimethylphenyl)carbonyl]-1-[(4-fluoropheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23706 (1-[(3,4-dichlorophenyl)carbonyl]-1H-pyrazole | N-B...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23701 (4-chloro-1-[(3-nitrophenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23710 (1-[(4-methylphenyl)carbonyl]-3-nitro-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23712 (1-[(2-methylphenyl)carbonyl]-4-nitro-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23701 (4-chloro-1-[(3-nitrophenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23704 (1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide | N-B...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23701 (4-chloro-1-[(3-nitrophenyl)carbonyl]-1H-pyrazole |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23702 (3-[(2,4-dimethylphenyl)carbonyl]-1-[(4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Urokinase activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration ... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |