

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24347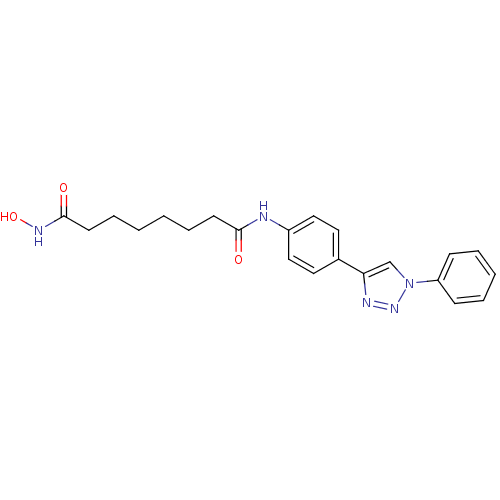 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24352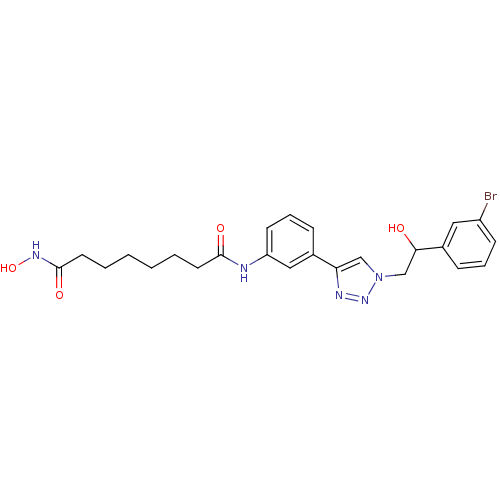 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24346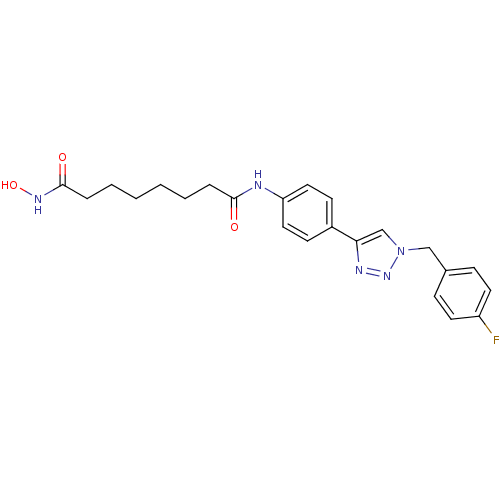 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24350 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24352 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24360 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24353 (N-[4-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24349 (N-[3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24357 (N-[3-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24355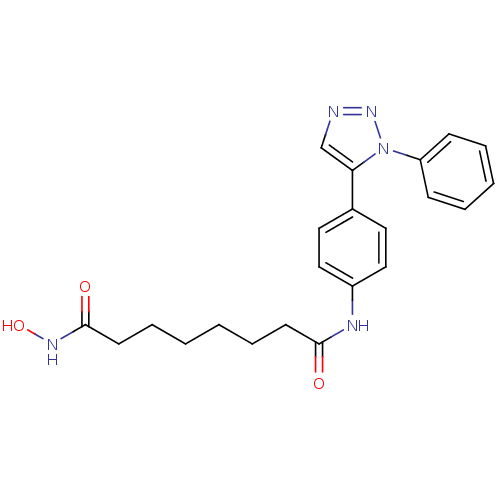 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-5-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24358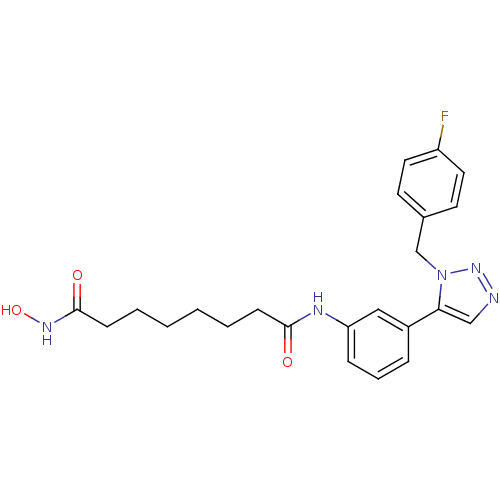 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24350 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24356 (N-(4-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24360 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24348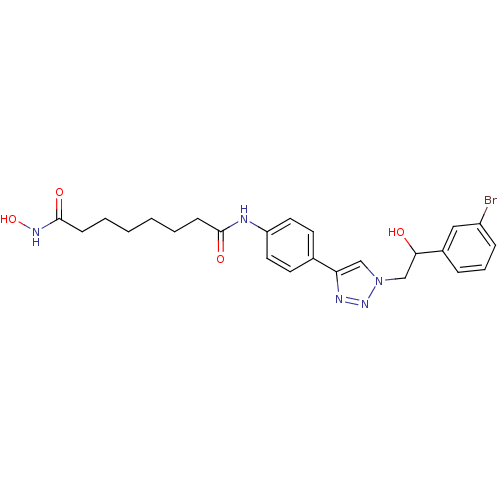 (N-(4-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24357 (N-[3-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24345 (N-[4-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24358 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24351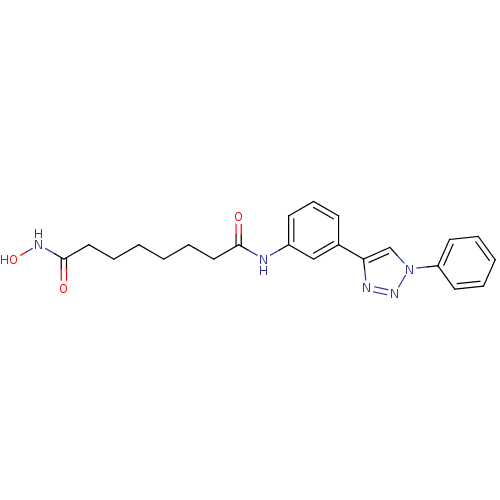 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24354 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24359 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-5-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24359 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-5-yl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24349 (N-[3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24347 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24351 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24353 (N-[4-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24355 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-5-yl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24352 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24346 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24349 (N-[3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24352 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24356 (N-(4-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24360 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24354 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24358 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24350 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24351 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24349 (N-[3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24357 (N-[3-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24357 (N-[3-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24360 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24359 (N-hydroxy-N'-[3-(1-phenyl-1H-1,2,3-triazol-5-yl)ph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24348 (N-(4-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24358 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24347 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |