 Found 36 hits Enz. Inhib. hit(s) with all data for entry = 22
Found 36 hits Enz. Inhib. hit(s) with all data for entry = 22 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387130

(4-ureidophenyl sulfamate ring derivative 3o | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2ccc(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H10F3N3O4S/c14-9-5-6-10(12(16)11(9)15)19-13(20)18-7-1-3-8(4-2-7)23-24(17,21)22/h1-6H,(H2,17,21,22)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387128
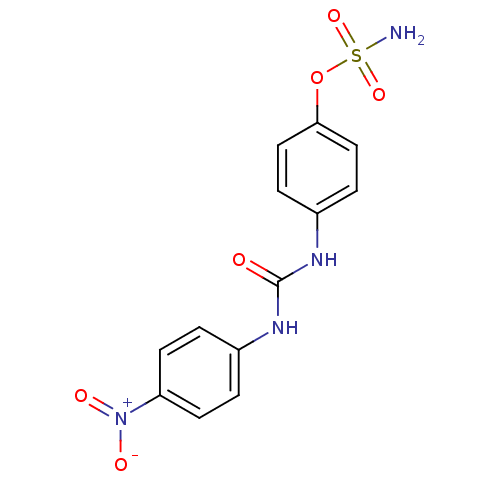
(4-ureidophenyl sulfamate ring derivative 3m | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C13H12N4O6S/c14-24(21,22)23-12-7-3-10(4-8-12)16-13(18)15-9-1-5-11(6-2-9)17(19)20/h1-8H,(H2,14,21,22)(H2,15,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387157
(4-ureidophenyl sulfamate ring derivative 3az | CHE...)Show InChI InChI=1S/C15H15N3O6S/c16-25(20,21)24-12-4-1-10(2-5-12)17-15(19)18-11-3-6-13-14(9-11)23-8-7-22-13/h1-6,9H,7-8H2,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387125
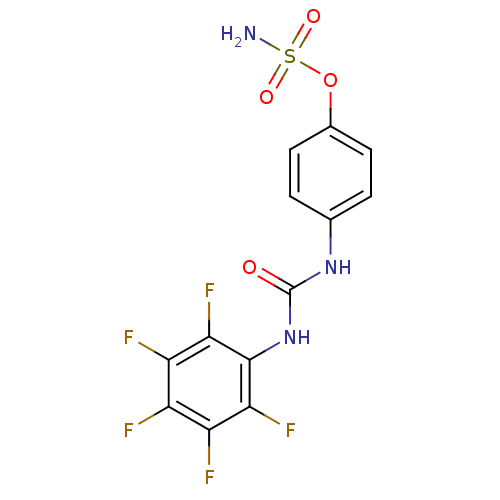
(4-ureidophenyl sulfamate ring derivative 3j | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H8F5N3O4S/c14-7-8(15)10(17)12(11(18)9(7)16)21-13(22)20-5-1-3-6(4-2-5)25-26(19,23)24/h1-4H,(H2,19,23,24)(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387155
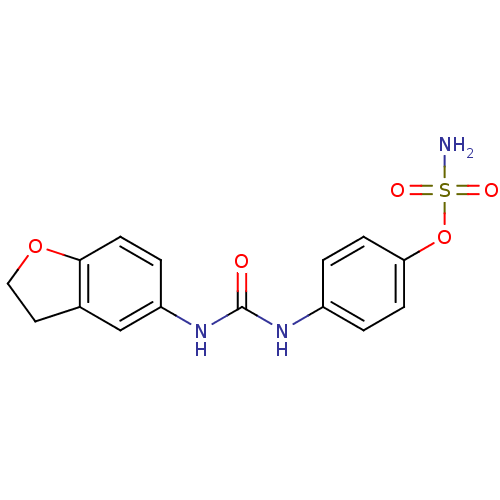
(4-ureidophenyl sulfamate ring derivative 3as | CHE...)Show InChI InChI=1S/C15H15N3O5S/c16-24(20,21)23-13-4-1-11(2-5-13)17-15(19)18-12-3-6-14-10(9-12)7-8-22-14/h1-6,9H,7-8H2,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387131
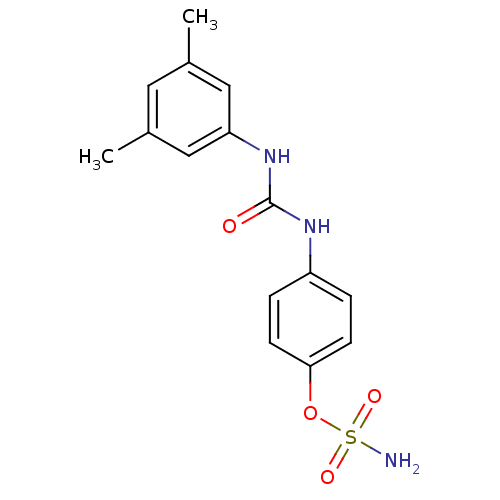
(4-ureidophenyl sulfamate ring derivative 3p | CHEM...)Show InChI InChI=1S/C15H17N3O4S/c1-10-7-11(2)9-13(8-10)18-15(19)17-12-3-5-14(6-4-12)22-23(16,20)21/h3-9H,1-2H3,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387161
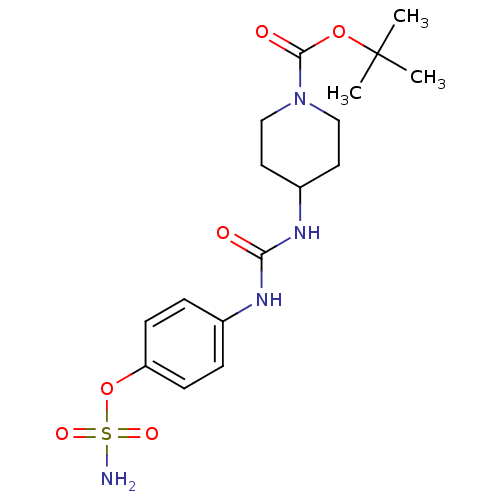
(4-ureidophenyl sulfamate ring derivative 3aw | CHE...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OS(N)(=O)=O)cc1 Show InChI InChI=1S/C17H26N4O6S/c1-17(2,3)26-16(23)21-10-8-13(9-11-21)20-15(22)19-12-4-6-14(7-5-12)27-28(18,24)25/h4-7,13H,8-11H2,1-3H3,(H2,18,24,25)(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387154
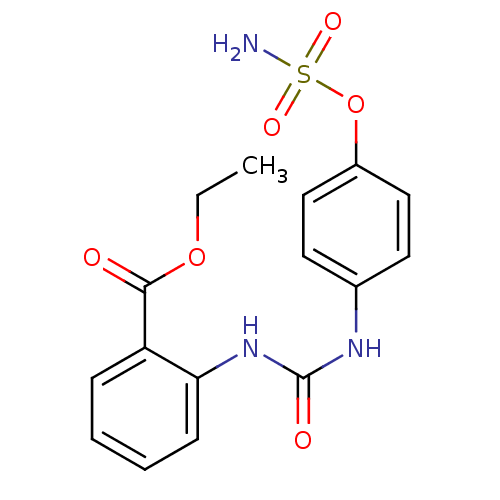
(4-ureidophenyl sulfamate ring derivative 3ar | CHE...)Show SMILES CCOC(=O)c1ccccc1NC(=O)Nc1ccc(OS(N)(=O)=O)cc1 Show InChI InChI=1S/C16H17N3O6S/c1-2-24-15(20)13-5-3-4-6-14(13)19-16(21)18-11-7-9-12(10-8-11)25-26(17,22)23/h3-10H,2H2,1H3,(H2,17,22,23)(H2,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387116
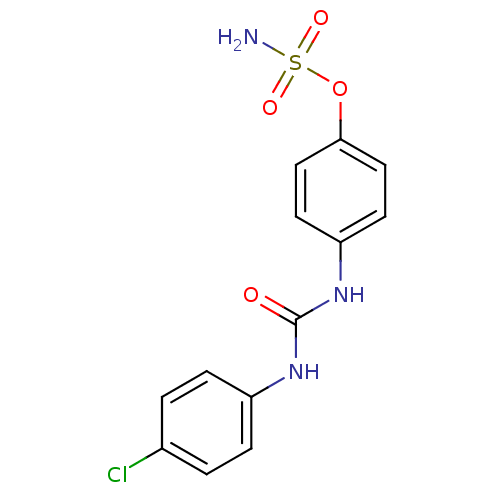
(4-ureidophenyl sulfamate ring derivative 3x | CHEM...)Show InChI InChI=1S/C13H12ClN3O4S/c14-9-1-3-10(4-2-9)16-13(18)17-11-5-7-12(8-6-11)21-22(15,19)20/h1-8H,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387132
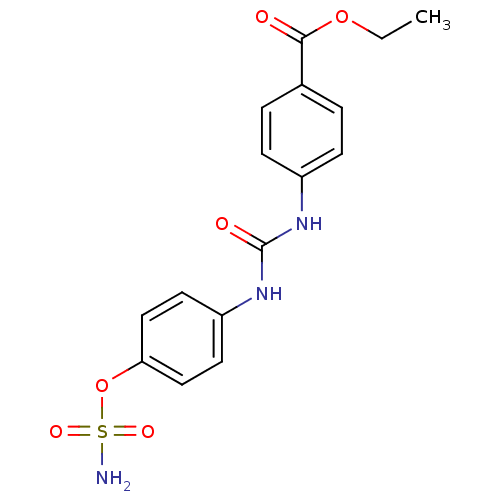
(4-ureidophenyl sulfamate ring derivative 3q | CHEM...)Show SMILES CCOC(=O)c1ccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)cc1 Show InChI InChI=1S/C16H17N3O6S/c1-2-24-15(20)11-3-5-12(6-4-11)18-16(21)19-13-7-9-14(10-8-13)25-26(17,22)23/h3-10H,2H2,1H3,(H2,17,22,23)(H2,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387129
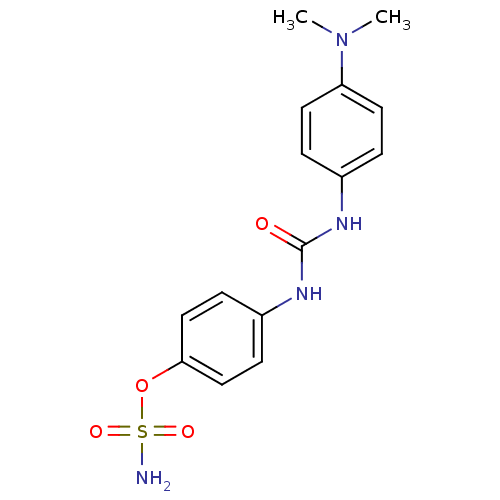
(4-ureidophenyl sulfamate ring derivative 3n | CHEM...)Show SMILES CN(C)c1ccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)cc1 Show InChI InChI=1S/C15H18N4O4S/c1-19(2)13-7-3-11(4-8-13)17-15(20)18-12-5-9-14(10-6-12)23-24(16,21)22/h3-10H,1-2H3,(H2,16,21,22)(H2,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387152
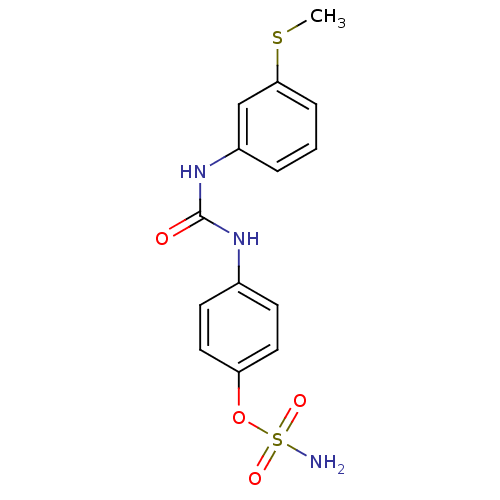
(4-ureidophenyl sulfamate ring derivative 3ap | CHE...)Show InChI InChI=1S/C14H15N3O4S2/c1-22-13-4-2-3-11(9-13)17-14(18)16-10-5-7-12(8-6-10)21-23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387134
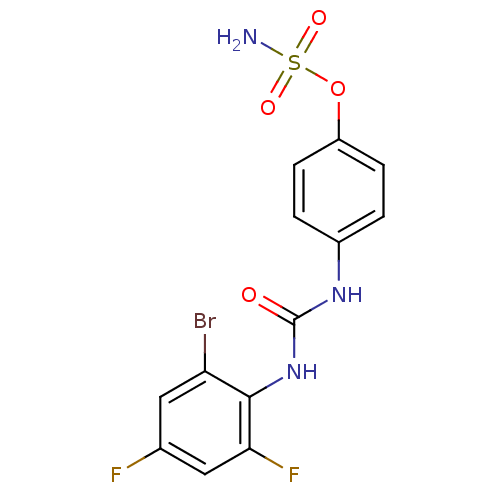
(4-ureidophenyl sulfamate ring derivative 3s | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(F)cc(F)cc2Br)cc1 Show InChI InChI=1S/C13H10BrF2N3O4S/c14-10-5-7(15)6-11(16)12(10)19-13(20)18-8-1-3-9(4-2-8)23-24(17,21)22/h1-6H,(H2,17,21,22)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387160
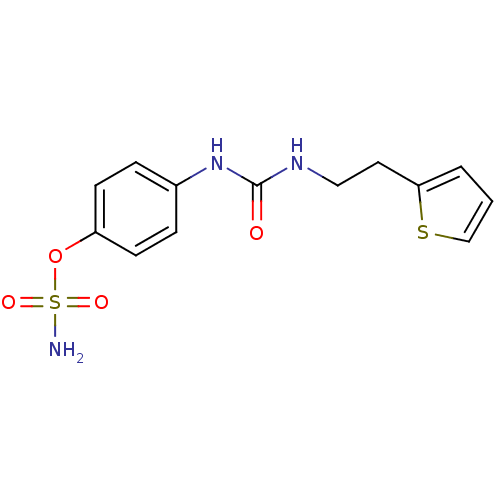
(4-ureidophenyl sulfamate ring derivative 3ay | CHE...)Show InChI InChI=1S/C13H15N3O4S2/c14-22(18,19)20-11-5-3-10(4-6-11)16-13(17)15-8-7-12-2-1-9-21-12/h1-6,9H,7-8H2,(H2,14,18,19)(H2,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387122
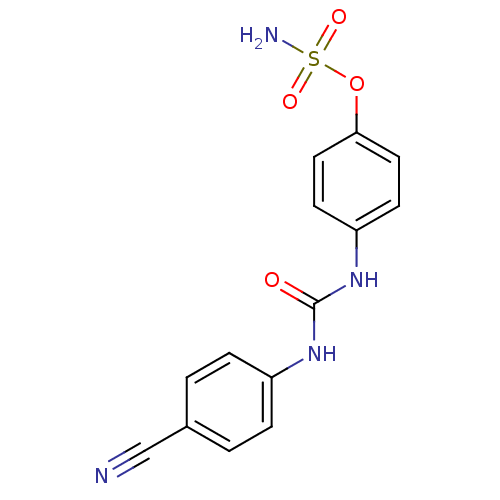
(4-ureidophenyl sulfamate ring derivative 3f | CHEM...)Show InChI InChI=1S/C14H12N4O4S/c15-9-10-1-3-11(4-2-10)17-14(19)18-12-5-7-13(8-6-12)22-23(16,20)21/h1-8H,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387137
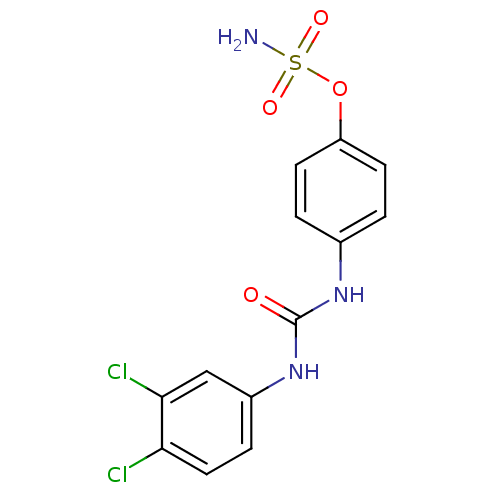
(4-ureidophenyl sulfamate ring derivative 3v | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C13H11Cl2N3O4S/c14-11-6-3-9(7-12(11)15)18-13(19)17-8-1-4-10(5-2-8)22-23(16,20)21/h1-7H,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387140
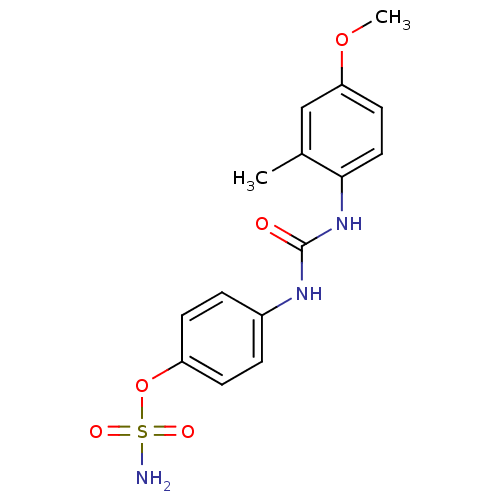
(4-ureidophenyl sulfamate ring derivative 3z | CHEM...)Show SMILES COc1ccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)c(C)c1 Show InChI InChI=1S/C15H17N3O5S/c1-10-9-13(22-2)7-8-14(10)18-15(19)17-11-3-5-12(6-4-11)23-24(16,20)21/h3-9H,1-2H3,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387163
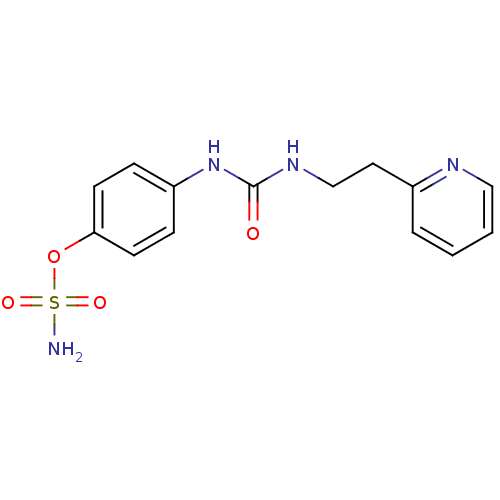
(4-ureidophenyl sulfamate ring derivative 3bb | CHE...)Show InChI InChI=1S/C14H16N4O4S/c15-23(20,21)22-13-6-4-12(5-7-13)18-14(19)17-10-8-11-3-1-2-9-16-11/h1-7,9H,8,10H2,(H2,15,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387121

(4-ureidophenyl sulfamate ring derivative 3e | CHEM...)Show InChI InChI=1S/C13H12IN3O4S/c14-9-1-3-10(4-2-9)16-13(18)17-11-5-7-12(8-6-11)21-22(15,19)20/h1-8H,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387118
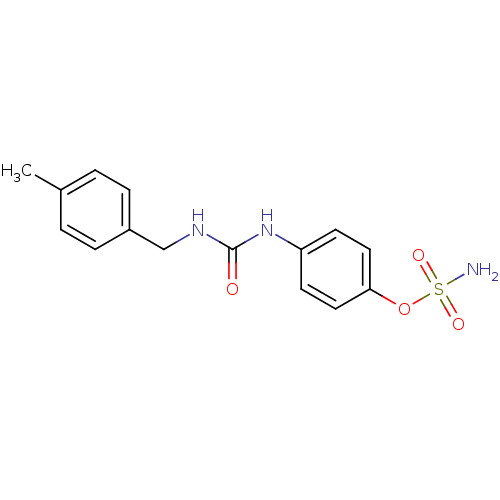
(4-ureidophenyl sulfamate ring derivative 3aj | CHE...)Show InChI InChI=1S/C15H17N3O4S/c1-11-2-4-12(5-3-11)10-17-15(19)18-13-6-8-14(9-7-13)22-23(16,20)21/h2-9H,10H2,1H3,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387135
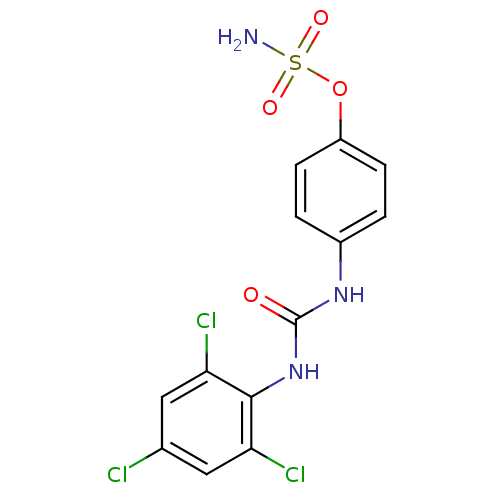
(4-ureidophenyl sulfamate ring derivative 3t | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(Cl)cc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C13H10Cl3N3O4S/c14-7-5-10(15)12(11(16)6-7)19-13(20)18-8-1-3-9(4-2-8)23-24(17,21)22/h1-6H,(H2,17,21,22)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387116

(4-ureidophenyl sulfamate ring derivative 3x | CHEM...)Show InChI InChI=1S/C13H12ClN3O4S/c14-9-1-3-10(4-2-9)16-13(18)17-11-5-7-12(8-6-11)21-22(15,19)20/h1-8H,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387162
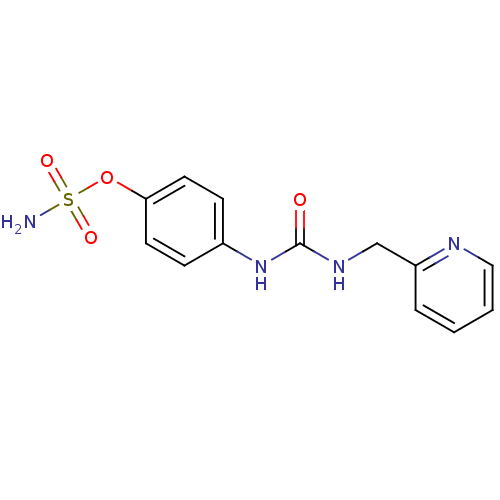
(4-ureidophenyl sulfamate ring derivative 3ba | CHE...)Show InChI InChI=1S/C13H14N4O4S/c14-22(19,20)21-12-6-4-10(5-7-12)17-13(18)16-9-11-3-1-2-8-15-11/h1-8H,9H2,(H2,14,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387156
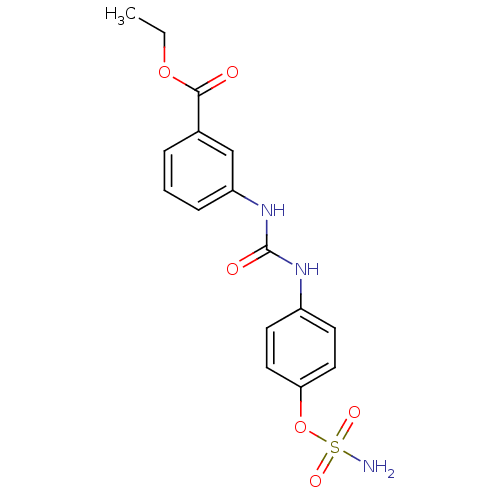
(4-ureidophenyl sulfamate ring derivative 3at | CHE...)Show SMILES CCOC(=O)c1cccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)c1 Show InChI InChI=1S/C16H17N3O6S/c1-2-24-15(20)11-4-3-5-13(10-11)19-16(21)18-12-6-8-14(9-7-12)25-26(17,22)23/h3-10H,2H2,1H3,(H2,17,22,23)(H2,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387120
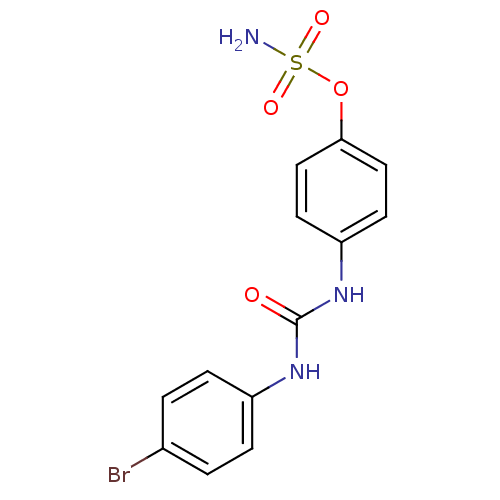
(4-ureidophenyl sulfamate ring derivative 3d | CHEM...)Show InChI InChI=1S/C13H12BrN3O4S/c14-9-1-3-10(4-2-9)16-13(18)17-11-5-7-12(8-6-11)21-22(15,19)20/h1-8H,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM65579

(4-ureidophenyl sulfamate ring derivative 3ae)Show SMILES COC(=O)c1ccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)cc1 Show InChI InChI=1S/C15H15N3O6S/c1-23-14(19)10-2-4-11(5-3-10)17-15(20)18-12-6-8-13(9-7-12)24-25(16,21)22/h2-9H,1H3,(H2,16,21,22)(H2,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387159
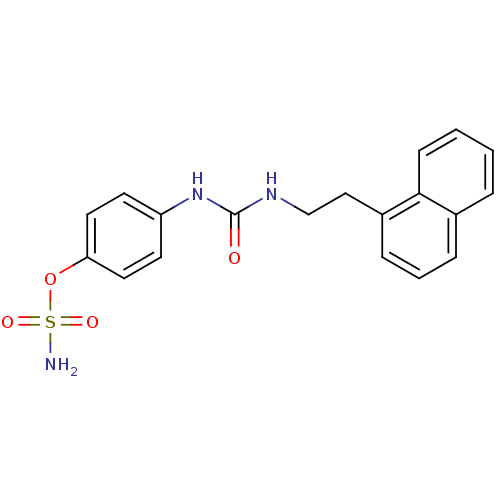
(4-ureidophenyl sulfamate ring derivative 3ax | CHE...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)NCCc2cccc3ccccc23)cc1 Show InChI InChI=1S/C19H19N3O4S/c20-27(24,25)26-17-10-8-16(9-11-17)22-19(23)21-13-12-15-6-3-5-14-4-1-2-7-18(14)15/h1-11H,12-13H2,(H2,20,24,25)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387117
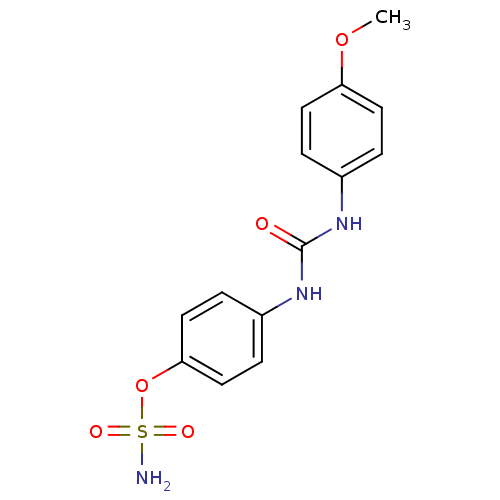
(4-ureidophenyl sulfamate ring derivative 3g | CHEM...)Show InChI InChI=1S/C14H15N3O5S/c1-21-12-6-2-10(3-7-12)16-14(18)17-11-4-8-13(9-5-11)22-23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
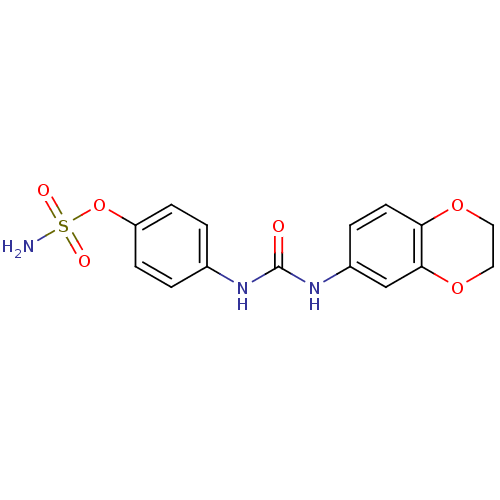
(Homo sapiens (Human)) | BDBM50387148
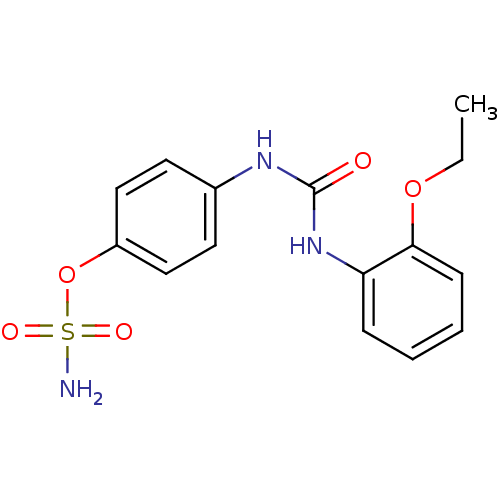
(4-ureidophenyl sulfamate ring derivative 3ai | CHE...)Show InChI InChI=1S/C15H17N3O5S/c1-2-22-14-6-4-3-5-13(14)18-15(19)17-11-7-9-12(10-8-11)23-24(16,20)21/h3-10H,2H2,1H3,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387127
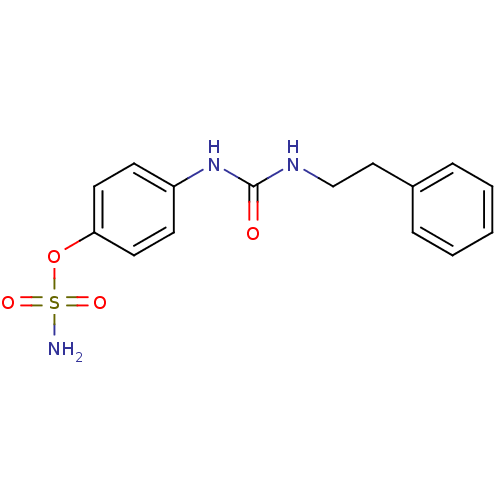
(4-ureidophenyl sulfamate ring derivative 3l | CHEM...)Show InChI InChI=1S/C15H17N3O4S/c16-23(20,21)22-14-8-6-13(7-9-14)18-15(19)17-11-10-12-4-2-1-3-5-12/h1-9H,10-11H2,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM65580
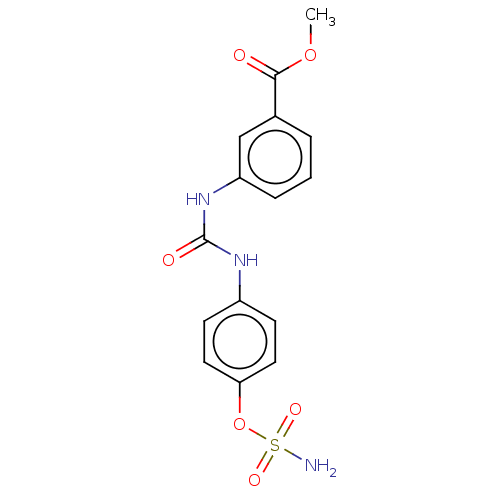
(4-ureidophenyl sulfamate ring derivative 3af)Show SMILES COC(=O)c1cccc(NC(=O)Nc2ccc(OS(N)(=O)=O)cc2)c1 Show InChI InChI=1S/C15H15N3O6S/c1-23-14(19)10-3-2-4-12(9-10)18-15(20)17-11-5-7-13(8-6-11)24-25(16,21)22/h2-9H,1H3,(H2,16,21,22)(H2,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387136
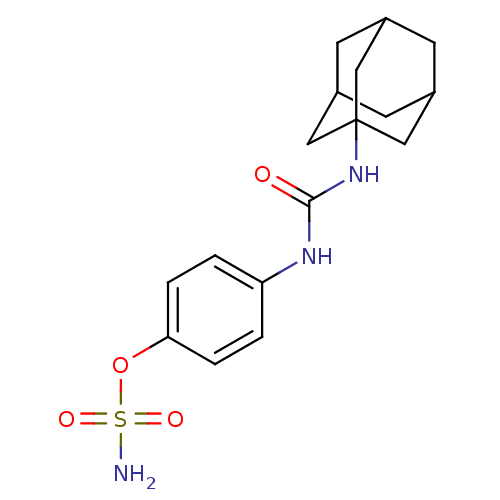
(4-ureidophenyl sulfamate ring derivative 3u | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| Show InChI InChI=1S/C17H23N3O4S/c18-25(22,23)24-15-3-1-14(2-4-15)19-16(21)20-17-8-11-5-12(9-17)7-13(6-11)10-17/h1-4,11-13H,5-10H2,(H2,18,22,23)(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387124
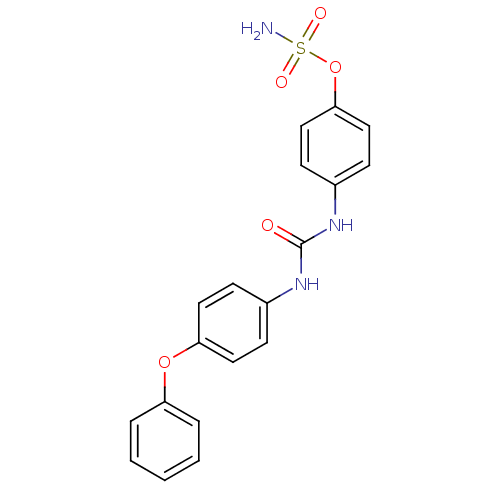
(4-ureidophenyl sulfamate ring derivative 3i | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C19H17N3O5S/c20-28(24,25)27-18-12-8-15(9-13-18)22-19(23)21-14-6-10-17(11-7-14)26-16-4-2-1-3-5-16/h1-13H,(H2,20,24,25)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387141
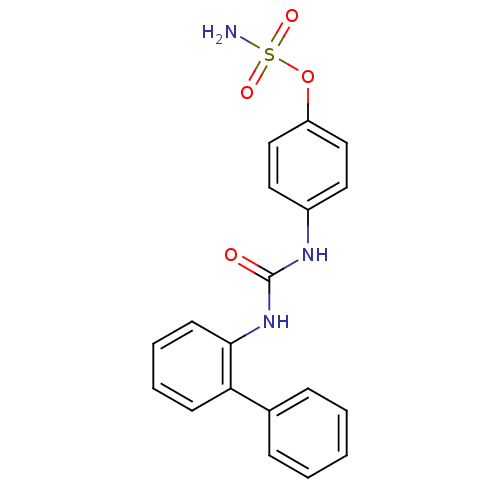
(4-ureidophenyl sulfamate ring derivative 3ab | CHE...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C19H17N3O4S/c20-27(24,25)26-16-12-10-15(11-13-16)21-19(23)22-18-9-5-4-8-17(18)14-6-2-1-3-7-14/h1-13H,(H2,20,24,25)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387119
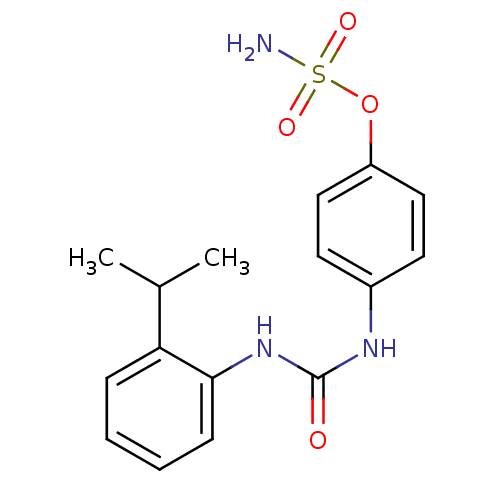
(4-ureidophenyl sulfamate ring derivative 3an | CHE...)Show InChI InChI=1S/C16H19N3O4S/c1-11(2)14-5-3-4-6-15(14)19-16(20)18-12-7-9-13(10-8-12)23-24(17,21)22/h3-11H,1-2H3,(H2,17,21,22)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50387151
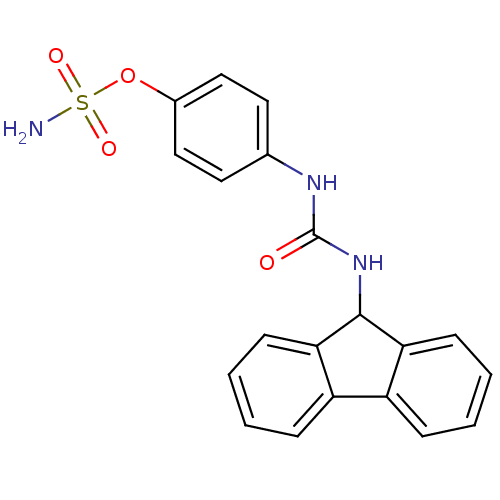
(4-ureidophenyl sulfamate ring derivative 3ao | CHE...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)NC2c3ccccc3-c3ccccc23)cc1 Show InChI InChI=1S/C20H17N3O4S/c21-28(25,26)27-14-11-9-13(10-12-14)22-20(24)23-19-17-7-3-1-5-15(17)16-6-2-4-8-18(16)19/h1-12,19H,(H2,21,25,26)(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
| |
J Enzyme Inhib Med Chem 29: 571-81 (2014)
Article DOI: 10.3109/14756366.2013.827677
BindingDB Entry DOI: 10.7270/Q2NC5ZCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data