

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25891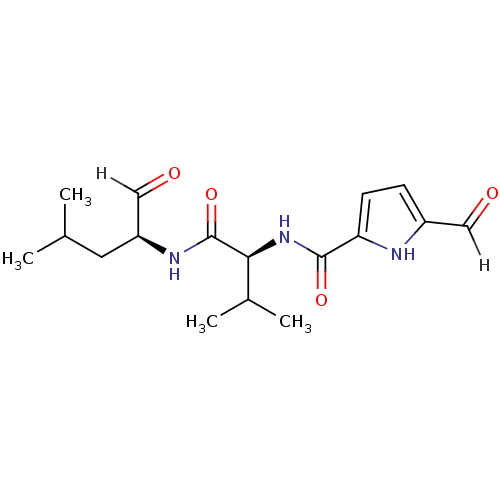 ((2S)-2-[(5-formyl-1H-pyrrol-2-yl)formamido]-3-meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25890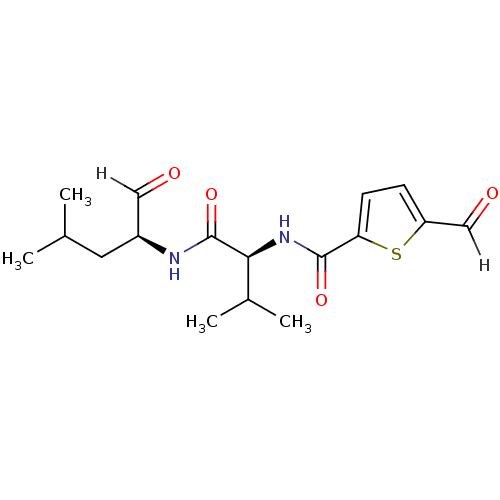 ((2S)-2-[(5-formylthiophen-2-yl)formamido]-3-methyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25892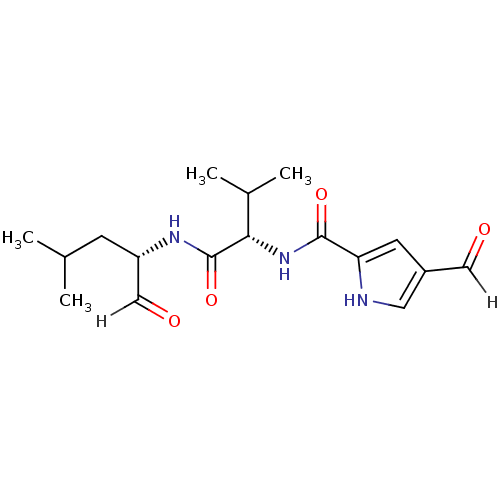 ((2S)-2-[(4-formyl-1H-pyrrol-2-yl)formamido]-3-meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25889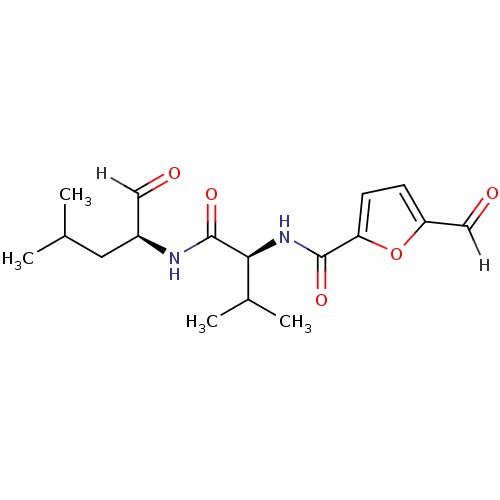 ((2S)-2-[(5-formylfuran-2-yl)formamido]-3-methyl-N-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25887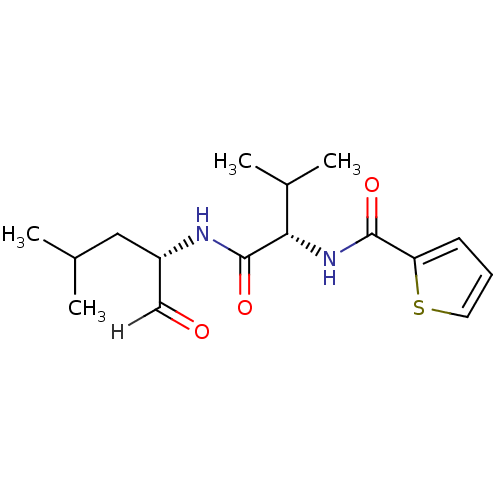 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25894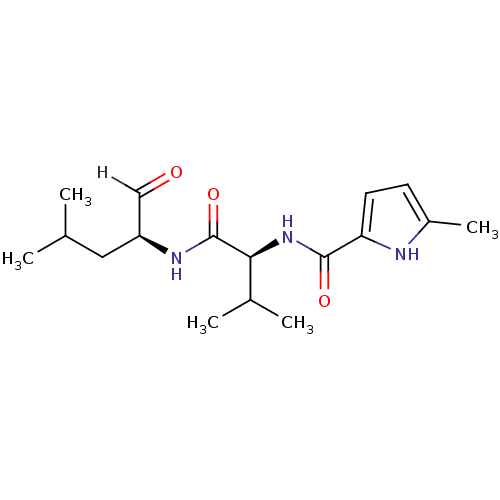 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25886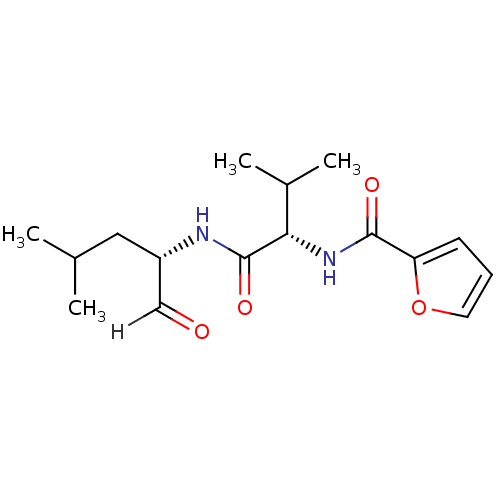 ((2S)-2-(furan-2-ylformamido)-3-methyl-N-[(2S)-4-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25895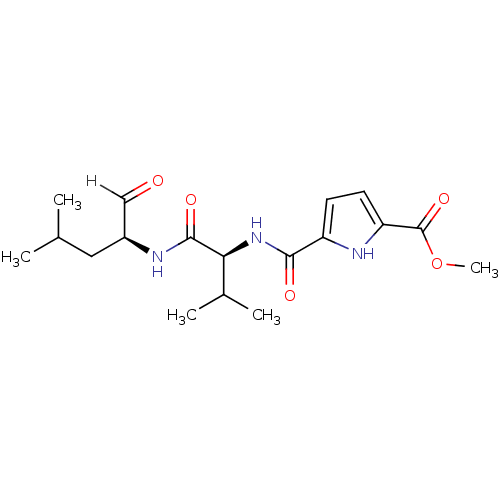 (N-heterocyclic dipeptide aldehyde, 13 | methyl 5-{...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25893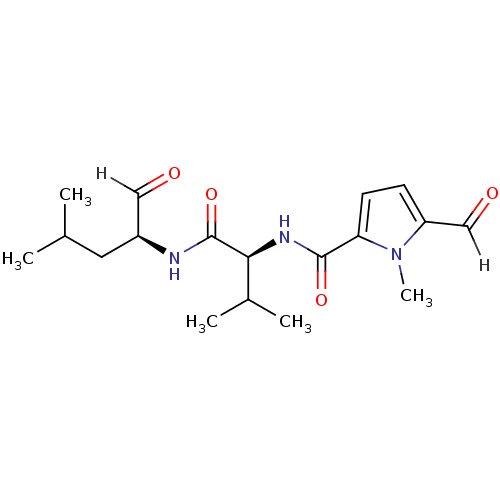 ((2S)-2-[(5-formyl-1-methyl-1H-pyrrol-2-yl)formamid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25893 ((2S)-2-[(5-formyl-1-methyl-1H-pyrrol-2-yl)formamid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25891 ((2S)-2-[(5-formyl-1H-pyrrol-2-yl)formamido]-3-meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25895 (N-heterocyclic dipeptide aldehyde, 13 | methyl 5-{...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-2 catalytic subunit (Ovis aries (sheep)) | BDBM25888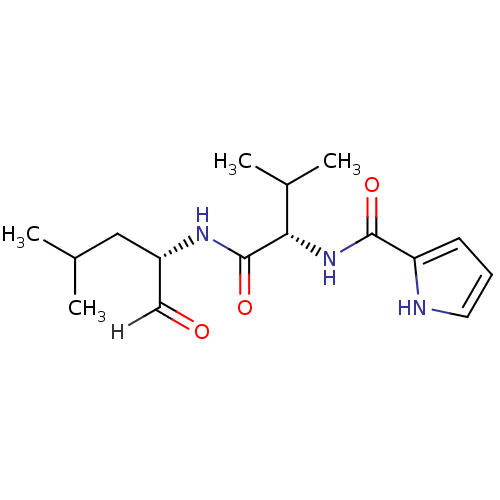 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25894 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25890 ((2S)-2-[(5-formylthiophen-2-yl)formamido]-3-methyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25892 ((2S)-2-[(4-formyl-1H-pyrrol-2-yl)formamido]-3-meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25888 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25887 ((2S)-3-methyl-N-[(2S)-4-methyl-1-oxopentan-2-yl]-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25886 ((2S)-2-(furan-2-ylformamido)-3-methyl-N-[(2S)-4-me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Ovis aries (sheep)) | BDBM25889 ((2S)-2-[(5-formylfuran-2-yl)formamido]-3-methyl-N-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Canterbury | Assay Description Calpain inhibition assays were performed in 96-well black plates. The reaction was initiated by the addition of substrate solution. The progress of t... | Bioorg Med Chem 16: 6911-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.048 BindingDB Entry DOI: 10.7270/Q2C53J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||