

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50107460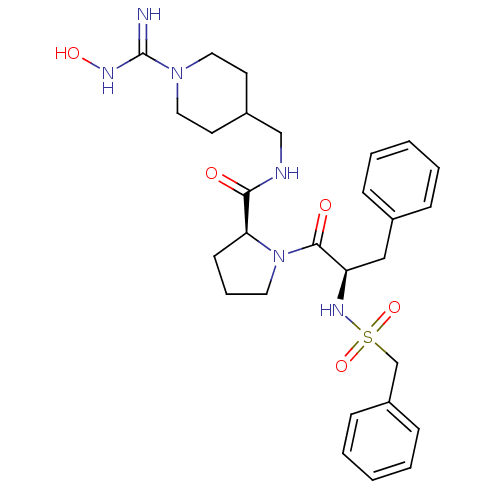 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107463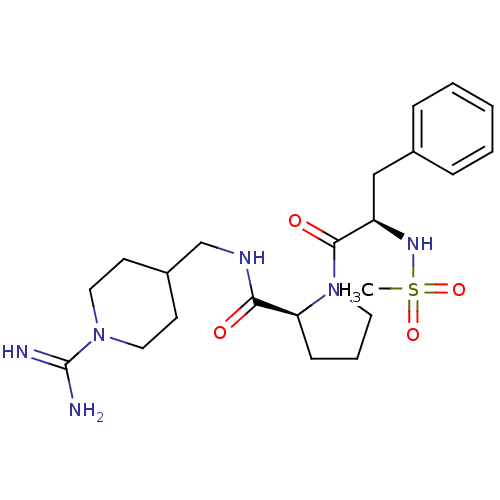 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for human alpha thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of thrombin was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107461 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001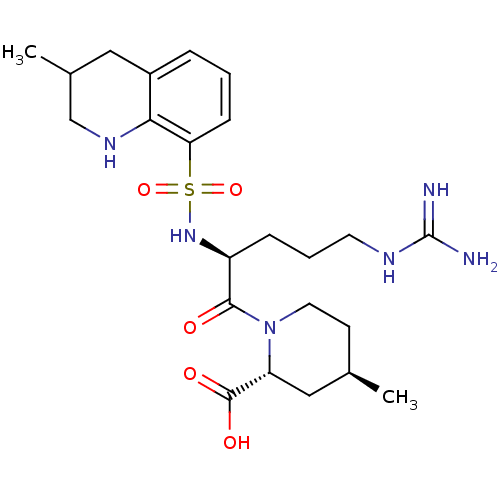 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107463 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107473 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50064652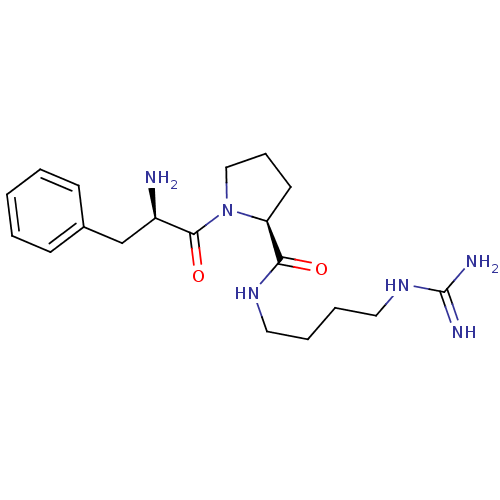 ((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107475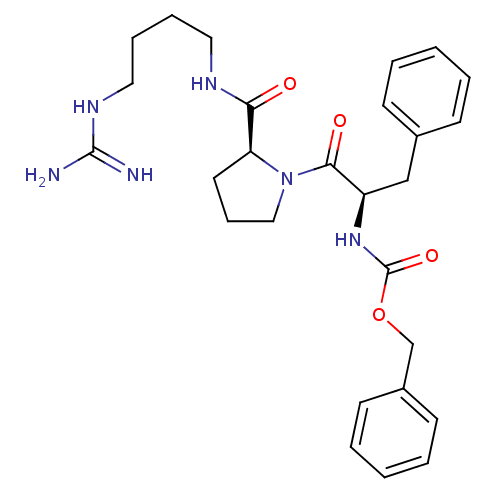 (CHEMBL148391 | {(R)-1-Benzyl-2-[(S)-2-(4-guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107458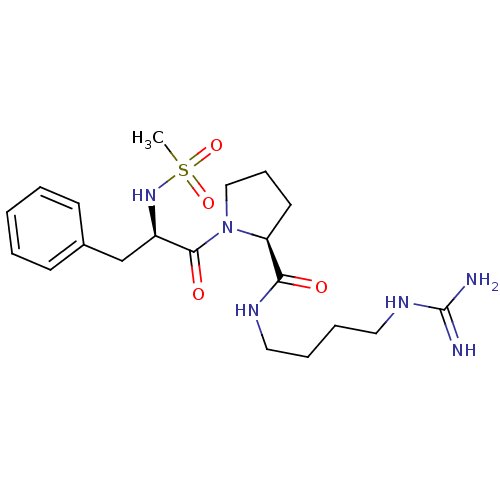 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107476 (1-[(S)-2-((S)-Naphthalene-2-sulfonylamino)-3-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107438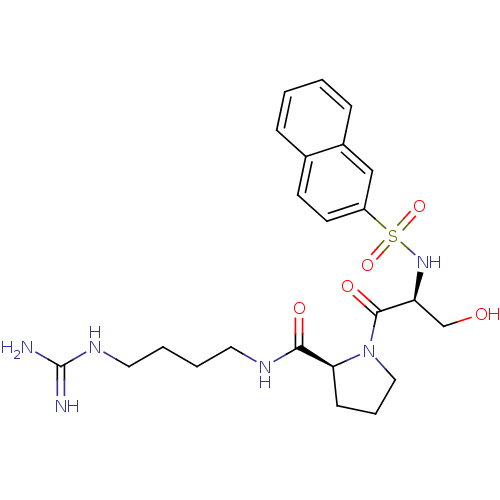 ((S)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107470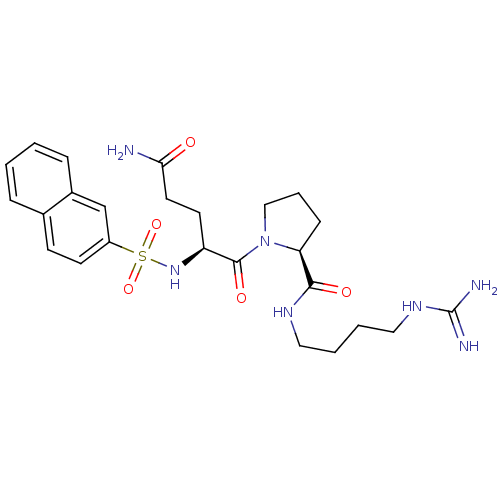 ((S)-1-[(S)-4-Carbamoyl-2-(naphthalene-2-sulfonylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107471 ((S)-1-[2-(Naphthalene-2-sulfonylamino)-acetyl]-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107462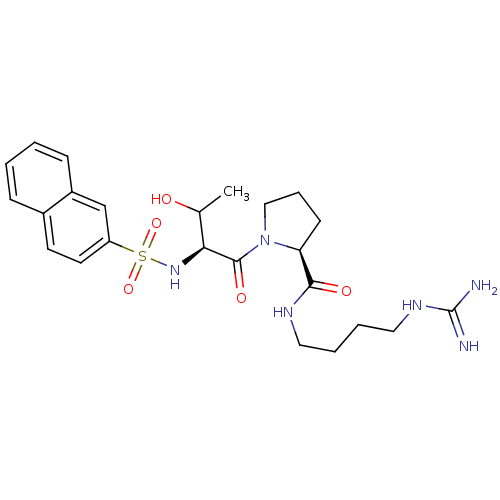 ((S)-1-[(2S,3S)-3-Hydroxy-2-(naphthalene-2-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107477 ((S)-1-[(2R,3R)-3-Methoxy-2-(naphthalene-2-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107472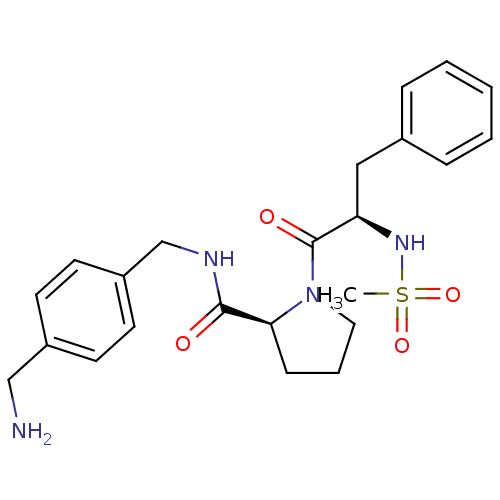 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107469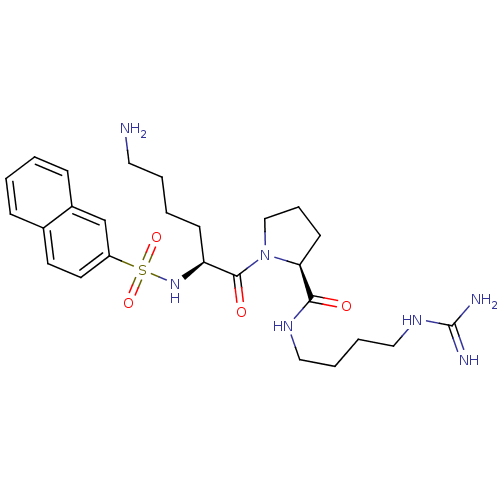 ((S)-1-[(S)-6-Amino-2-(naphthalene-2-sulfonylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107474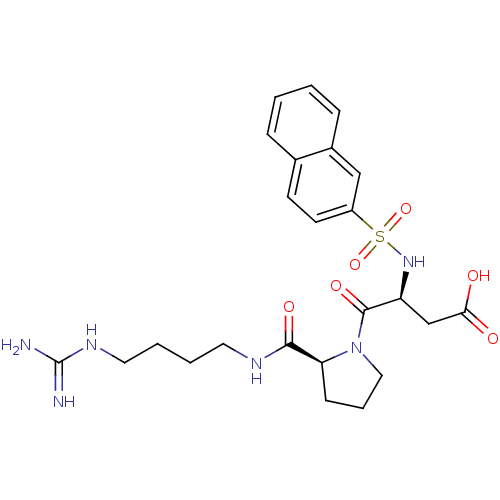 ((S)-4-[(S)-2-(4-Guanidino-butylcarbamoyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107459 ((S)-1-[(R)-3-(1H-Indol-3-yl)-2-(naphthalene-2-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107456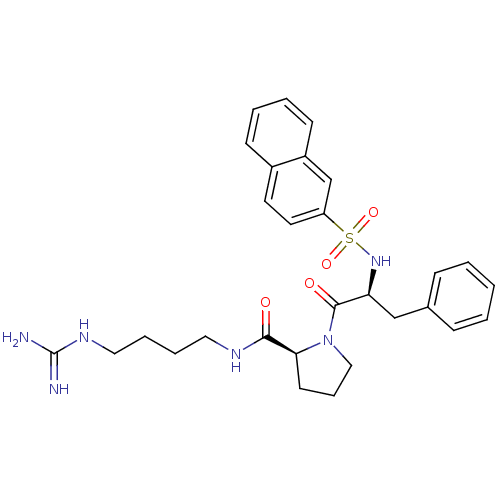 ((S)-1-[(S)-2-(Naphthalene-2-sulfonylamino)-3-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107468 ((S)-1-[4-Carbamoyl-2-((S)-naphthalene-2-sulfonylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107466 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107467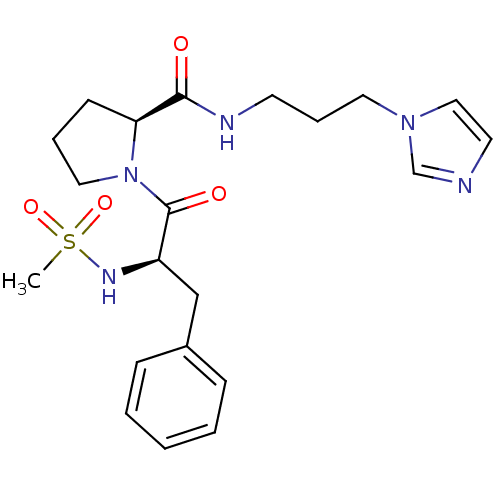 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107465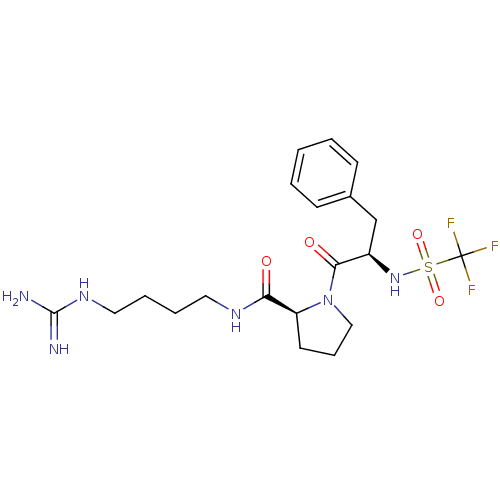 ((S)-1-((R)-3-Phenyl-2-trifluoromethanesulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107457 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107464 ((R)-5-[(S)-2-(4-Guanidino-butylcarbamoyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against hydrolysis of human alpha thrombin | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||