

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115967 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115966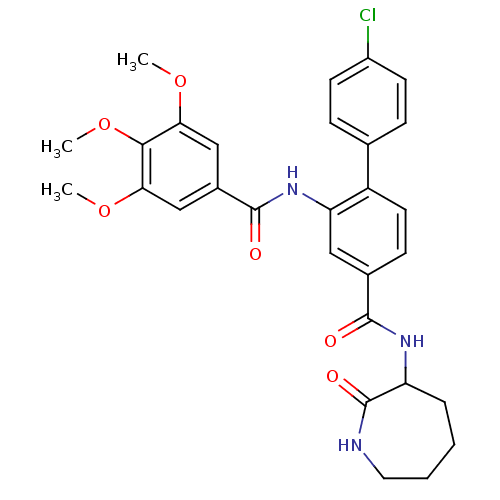 (4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403920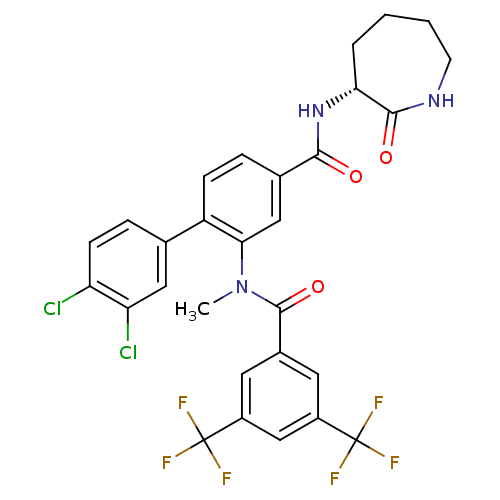 (CHEMBL2112212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403918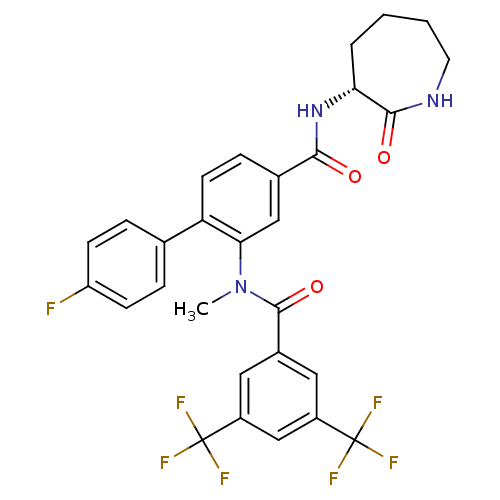 (CHEMBL2113739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115965 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115959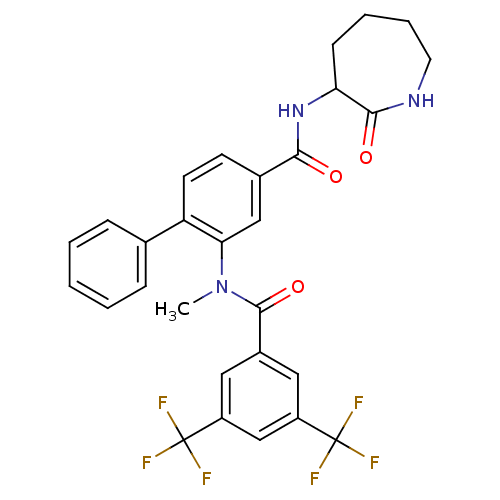 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403916 (CHEMBL2113740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115957 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115969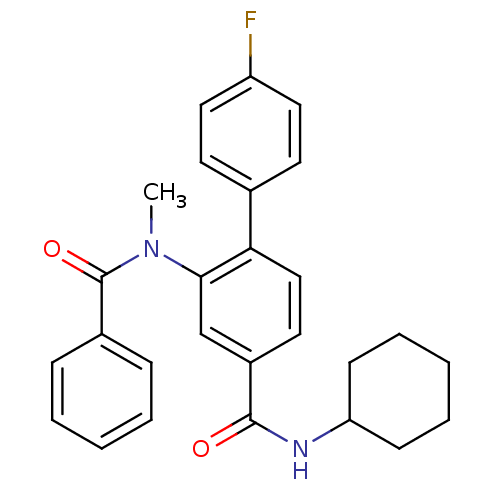 (CHEMBL305375 | N-[4-(N-Cyclohexyl-hydrazinocarbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115964 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403919 (CHEMBL2112211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115970 (4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50403917 (CHEMBL2112209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403918 (CHEMBL2113739) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115970 (4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50115963 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403920 (CHEMBL2112212) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115957 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403916 (CHEMBL2113740) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115965 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403917 (CHEMBL2112209) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115963 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403919 (CHEMBL2112211) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115964 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115959 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115967 (2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115969 (CHEMBL305375 | N-[4-(N-Cyclohexyl-hydrazinocarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50115966 (4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... | Bioorg Med Chem Lett 12: 2065-8 (2002) BindingDB Entry DOI: 10.7270/Q2K64HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||