

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119815 (3-chloro-4-[16-methyl-(12S)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119813 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119807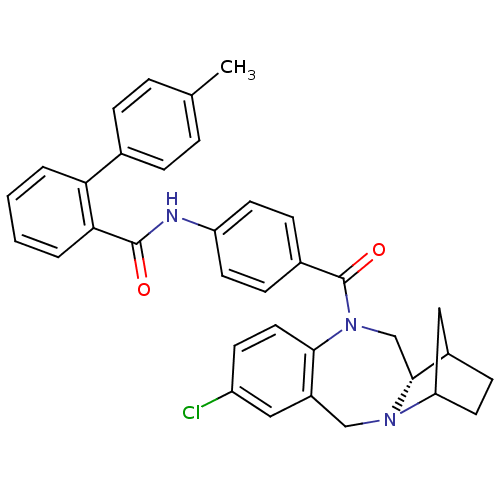 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119807 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119816 (3-chloro-4-[16-methyl-(12R)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119812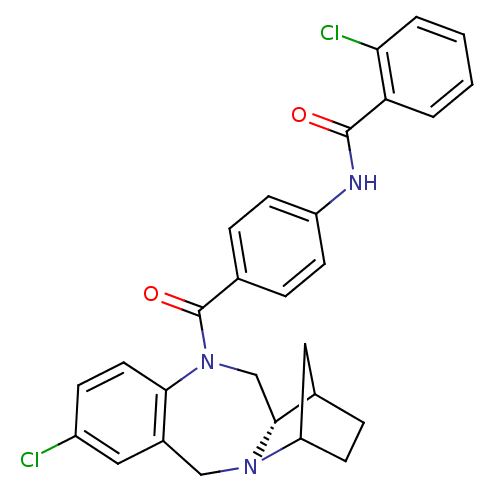 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119813 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119812 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119815 (3-chloro-4-[16-methyl-(12S)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119817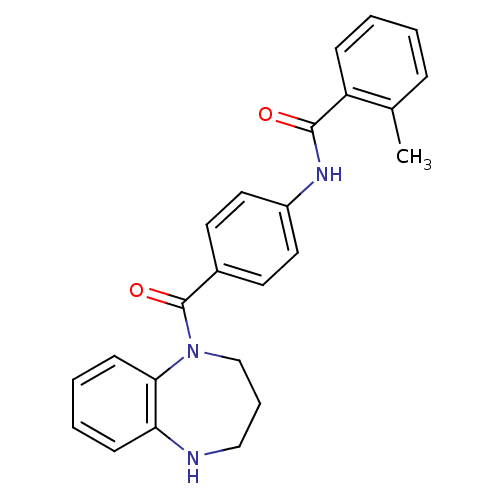 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b][1,4]dia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119810 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119811 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119811 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119817 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b][1,4]dia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119810 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119813 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119815 (3-chloro-4-[16-methyl-(12S)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119818 (3-chloro-4-[16-ethyl-2,10-diazatetracyclo[11.2.1.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119812 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119807 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119814 (1N-{4-[16-ethyl-2,10-diazatetracyclo[11.2.1.02,12....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119813 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119812 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119811 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119816 (3-chloro-4-[16-methyl-(12R)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119819 (3-chloro-4-[16-ethyl-(12S)-2,10-diazatetracyclo[11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119815 (3-chloro-4-[16-methyl-(12S)-2,10-diazatetracyclo[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119807 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119810 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119817 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b][1,4]dia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119809 (3-chloro-4-[6-chloro-16-methyl-(12S)-2,10-diazatet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119809 (3-chloro-4-[6-chloro-16-methyl-(12S)-2,10-diazatet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119811 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119808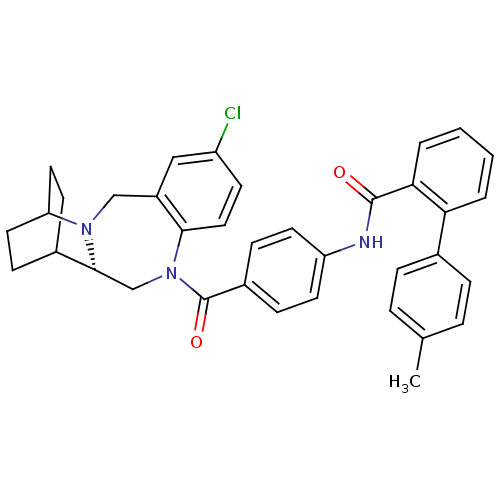 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119810 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V2 receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50119808 (1N-{4-[6-chloro-16-ethyl-(12S)-2,10-diazatetracycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]-Arg-vasopressin binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50119817 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b][1,4]dia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of AVP mediated activation of human vasopressin V1a receptor expressed in HEK-293 cells | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50119807 (1N-{4-[6-chloro-16-methyl-(12S)-2,10-diazatetracyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2C9 | Bioorg Med Chem Lett 12: 3081-4 (2002) BindingDB Entry DOI: 10.7270/Q22N51NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||