 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50001241
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50001241 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50014113
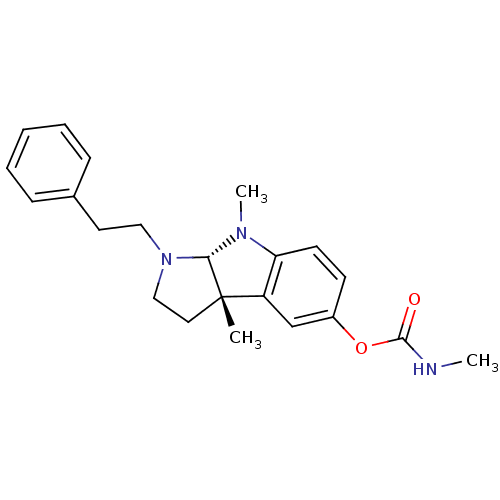
(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014110

(CHEMBL307721 | Methyl-carbamic acid 1-allyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CC=C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H23N3O2/c1-5-9-20-10-8-17(2)13-11-12(22-16(21)18-3)6-7-14(13)19(4)15(17)20/h5-7,11,15H,1,8-10H2,2-4H3,(H,18,21)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014111

(CHEMBL305670 | Methyl-carbamic acid 3a,8-dimethyl-...)Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-16-12(14)17(3)11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014113

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014112
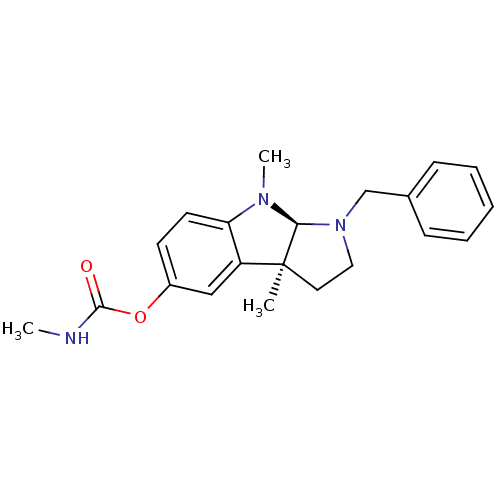
(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014110

(CHEMBL307721 | Methyl-carbamic acid 1-allyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CC=C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H23N3O2/c1-5-9-20-10-8-17(2)13-11-12(22-16(21)18-3)6-7-14(13)19(4)15(17)20/h5-7,11,15H,1,8-10H2,2-4H3,(H,18,21)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10958
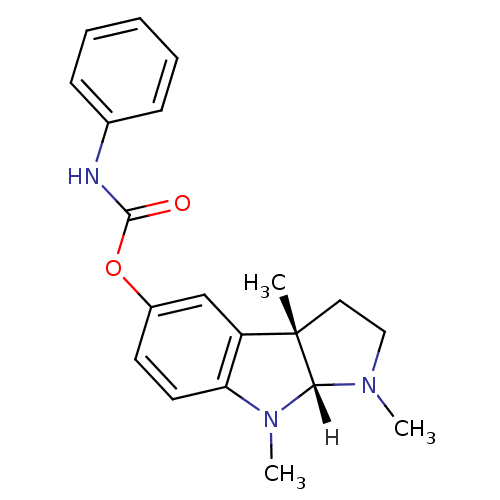
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014111

(CHEMBL305670 | Methyl-carbamic acid 3a,8-dimethyl-...)Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-16-12(14)17(3)11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014111

(CHEMBL305670 | Methyl-carbamic acid 3a,8-dimethyl-...)Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-16-12(14)17(3)11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014110

(CHEMBL307721 | Methyl-carbamic acid 1-allyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CC=C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H23N3O2/c1-5-9-20-10-8-17(2)13-11-12(22-16(21)18-3)6-7-14(13)19(4)15(17)20/h5-7,11,15H,1,8-10H2,2-4H3,(H,18,21)/t15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014111

(CHEMBL305670 | Methyl-carbamic acid 3a,8-dimethyl-...)Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-16-12(14)17(3)11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014110

(CHEMBL307721 | Methyl-carbamic acid 1-allyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CC=C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H23N3O2/c1-5-9-20-10-8-17(2)13-11-12(22-16(21)18-3)6-7-14(13)19(4)15(17)20/h5-7,11,15H,1,8-10H2,2-4H3,(H,18,21)/t15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014112

(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014111

(CHEMBL305670 | Methyl-carbamic acid 3a,8-dimethyl-...)Show InChI InChI=1S/C14H19N3O2/c1-14-6-7-16-12(14)17(3)11-5-4-9(8-10(11)14)19-13(18)15-2/h4-5,8,12,16H,6-7H2,1-3H3,(H,15,18)/t12-,14+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014110

(CHEMBL307721 | Methyl-carbamic acid 1-allyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CC=C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H23N3O2/c1-5-9-20-10-8-17(2)13-11-12(22-16(21)18-3)6-7-14(13)19(4)15(17)20/h5-7,11,15H,1,8-10H2,2-4H3,(H,18,21)/t15-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014107
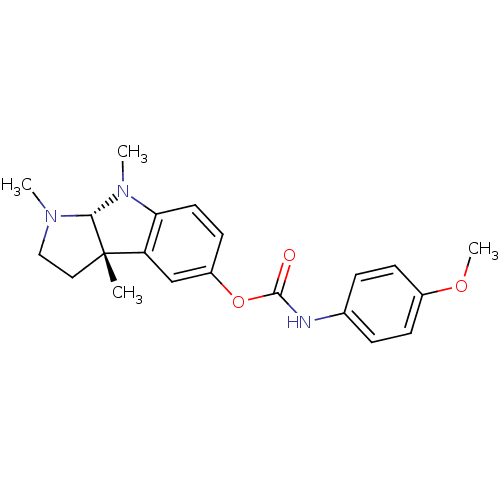
((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014113

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014112

(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10984
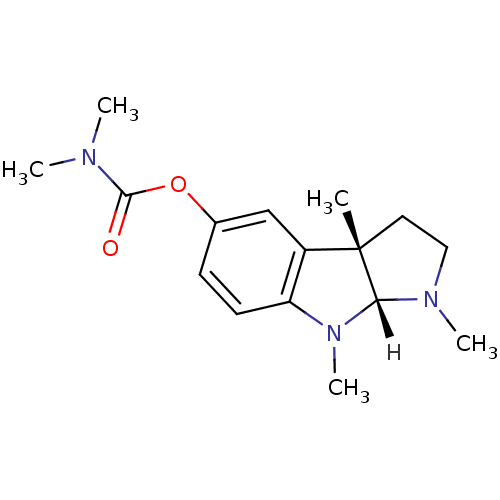
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)C)ccc1N2C |r| Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-18(4)14(16)19(5)13-7-6-11(10-12(13)16)21-15(20)17(2)3/h6-7,10,14H,8-9H2,1-5H3/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014113

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014107

((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014107

((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10984

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)C)ccc1N2C |r| Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-18(4)14(16)19(5)13-7-6-11(10-12(13)16)21-15(20)17(2)3/h6-7,10,14H,8-9H2,1-5H3/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014112

(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in erythrocyte |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014107

((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10984

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)C)ccc1N2C |r| Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-18(4)14(16)19(5)13-7-6-11(10-12(13)16)21-15(20)17(2)3/h6-7,10,14H,8-9H2,1-5H3/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10984

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)C)ccc1N2C |r| Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-18(4)14(16)19(5)13-7-6-11(10-12(13)16)21-15(20)17(2)3/h6-7,10,14H,8-9H2,1-5H3/t14-,16+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014113

(3alpha,8-dimethyl-1-phenethyl-1,2,3,3alpha,8,8alph...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(CCc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H27N3O2/c1-22-12-14-25(13-11-16-7-5-4-6-8-16)20(22)24(3)19-10-9-17(15-18(19)22)27-21(26)23-2/h4-10,15,20H,11-14H2,1-3H3,(H,23,26)/t20-,22-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014112

(CHEMBL74257 | Methyl-carbamic acid 1-benzyl-3a,8-d...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(Cc4ccccc4)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-24(14-15-7-5-4-6-8-15)19(21)23(3)18-10-9-16(13-17(18)21)26-20(25)22-2/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/t19-,21-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50014107

((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in electric eel |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50014108

((-)-tert-Butyl-carbamic acid 1,3a,8-trimethyl-1,2,...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NC(C)(C)C)cc21 Show InChI InChI=1S/C18H27N3O2/c1-17(2,3)19-16(22)23-12-7-8-14-13(11-12)18(4)9-10-20(5)15(18)21(14)6/h7-8,11,15H,9-10H2,1-6H3,(H,19,22)/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Butyrylcholinesterase in plasma |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acetylcholinesterase in cortex |
J Med Chem 33: 2311-9 (1990)
BindingDB Entry DOI: 10.7270/Q24M93HF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data