 Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50013594
Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50013594 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132318

(3-(1-{2-[2-(2-Dimethylamino-ethoxy)-ethoxy]-ethyl}...)Show SMILES CN1CCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCCOCC1)c1ccccc31)c1ccccc21 |t:8| Show InChI InChI=1S/C30H32N4O4/c1-32-11-6-12-33-19-23(21-7-2-4-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-5-3-8-22(24)26)14-16-38-18-17-37-15-13-32/h2-5,7-10,19-20H,6,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132309
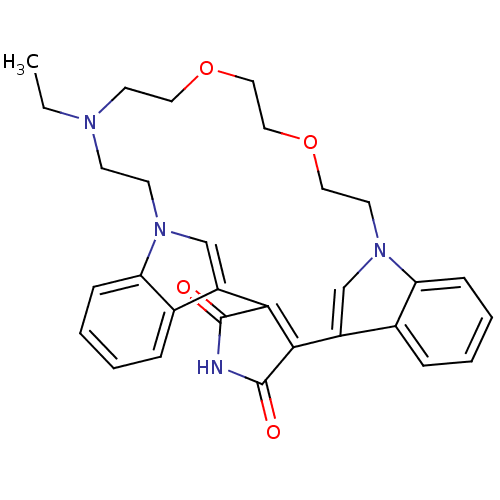
(23-ethyl-17,20-dioxa-4,14,23,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-12-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-6-4-8-22(24)26)14-16-38-18-17-37-15-13-32/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132317
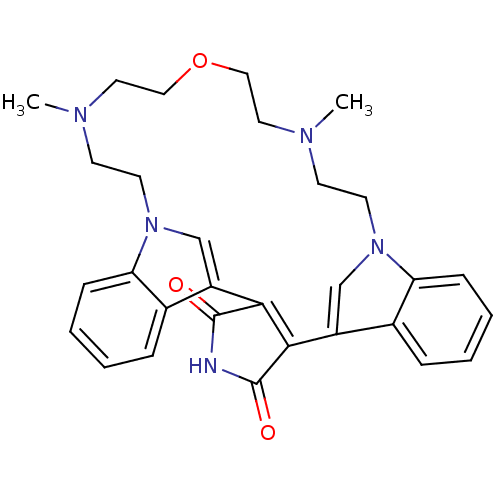
(17,23-dimethyl-20-oxa-4,14,17,23,26-pentaazahexacy...)Show SMILES CN1CCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H33N5O3/c1-32-11-13-34-19-23(21-7-3-5-9-25(21)34)27-28(30(37)31-29(27)36)24-20-35(26-10-6-4-8-22(24)26)14-12-33(2)16-18-38-17-15-32/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Calcium/calmodulin-dependent protein kinase type II |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
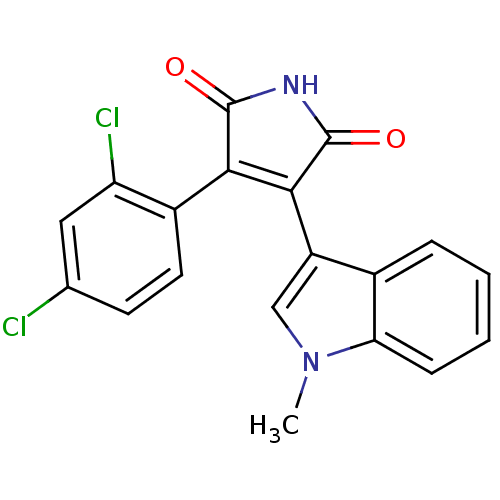
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132316
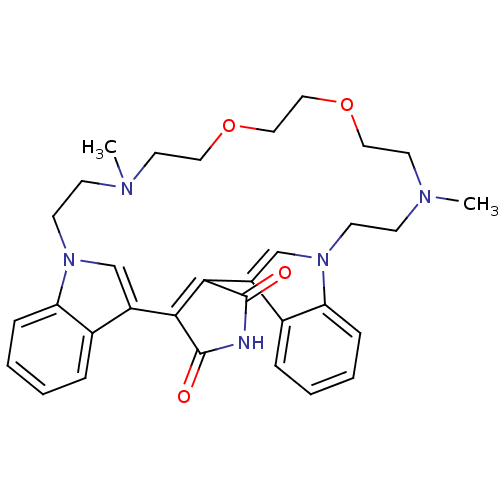
(17,26-dimethyl-20,23-dioxa-4,14,17,26,29-pentaazah...)Show SMILES CN1CCOCCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:17| Show InChI InChI=1S/C32H37N5O4/c1-34-11-13-36-21-25(23-7-3-5-9-27(23)36)29-30(32(39)33-31(29)38)26-22-37(28-10-6-4-8-24(26)28)14-12-35(2)16-18-41-20-19-40-17-15-34/h3-10,21-22H,11-20H2,1-2H3,(H,33,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132310
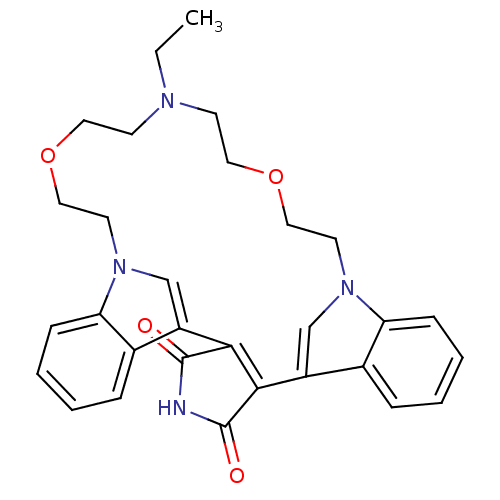
(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132311
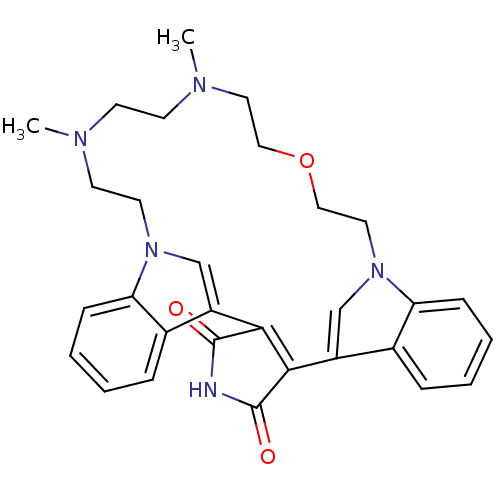
(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132319
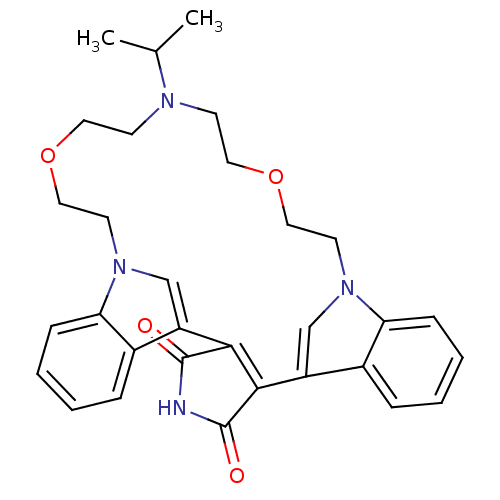
(20-isopropyl-17,23-dioxa-4,14,20,26-tetraazahexacy...)Show SMILES CC(C)N1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C31H34N4O4/c1-21(2)33-11-15-38-17-13-34-19-24(22-7-3-5-9-26(22)34)28-29(31(37)32-30(28)36)25-20-35(14-18-39-16-12-33)27-10-6-4-8-23(25)27/h3-10,19-21H,11-18H2,1-2H3,(H,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132309

(23-ethyl-17,20-dioxa-4,14,23,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-12-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-6-4-8-22(24)26)14-16-38-18-17-37-15-13-32/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132317

(17,23-dimethyl-20-oxa-4,14,17,23,26-pentaazahexacy...)Show SMILES CN1CCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H33N5O3/c1-32-11-13-34-19-23(21-7-3-5-9-25(21)34)27-28(30(37)31-29(27)36)24-20-35(26-10-6-4-8-22(24)26)14-12-33(2)16-18-38-17-15-32/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132315
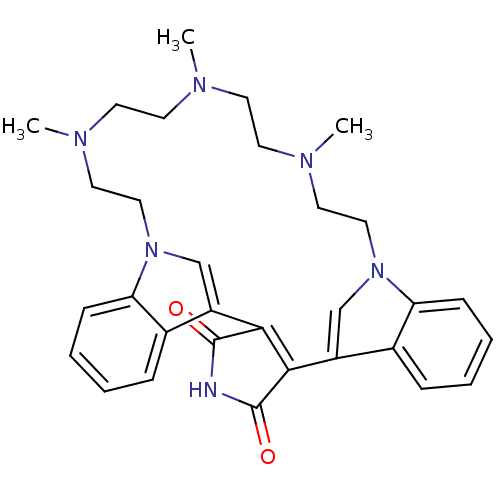
(17,20,23-trimethyl-4,14,17,20,23,26-hexaazahexacyc...)Show SMILES CN1CCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C31H36N6O2/c1-33-12-14-34(2)16-18-36-20-24(22-8-4-6-10-26(22)36)28-29(31(39)32-30(28)38)25-21-37(19-17-35(3)15-13-33)27-11-7-5-9-23(25)27/h4-11,20-21H,12-19H2,1-3H3,(H,32,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132309

(23-ethyl-17,20-dioxa-4,14,23,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-12-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-6-4-8-22(24)26)14-16-38-18-17-37-15-13-32/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132315

(17,20,23-trimethyl-4,14,17,20,23,26-hexaazahexacyc...)Show SMILES CN1CCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C31H36N6O2/c1-33-12-14-34(2)16-18-36-20-24(22-8-4-6-10-26(22)36)28-29(31(39)32-30(28)38)25-21-37(19-17-35(3)15-13-33)27-11-7-5-9-23(25)27/h4-11,20-21H,12-19H2,1-3H3,(H,32,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132315

(17,20,23-trimethyl-4,14,17,20,23,26-hexaazahexacyc...)Show SMILES CN1CCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C31H36N6O2/c1-33-12-14-34(2)16-18-36-20-24(22-8-4-6-10-26(22)36)28-29(31(39)32-30(28)38)25-21-37(19-17-35(3)15-13-33)27-11-7-5-9-23(25)27/h4-11,20-21H,12-19H2,1-3H3,(H,32,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132314
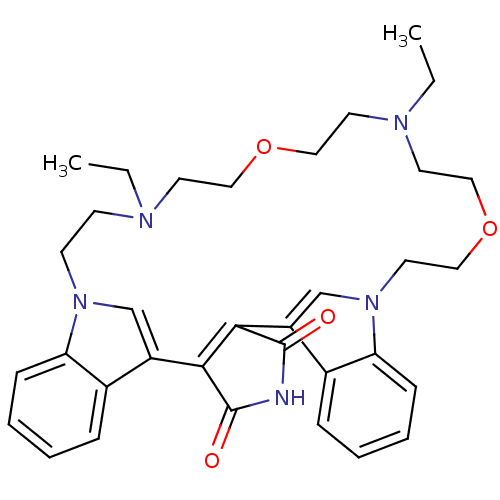
(20,26-diethyl-17,23-dioxa-4,14,20,26,29-pentaazahe...)Show SMILES CCN1CCOCCN(CC)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:16| Show InChI InChI=1S/C34H41N5O4/c1-3-36-13-14-38-23-27(25-9-5-7-11-29(25)38)31-32(34(41)35-33(31)40)28-24-39(30-12-8-6-10-26(28)30)18-22-43-21-17-37(4-2)16-20-42-19-15-36/h5-12,23-24H,3-4,13-22H2,1-2H3,(H,35,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132311

(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132308
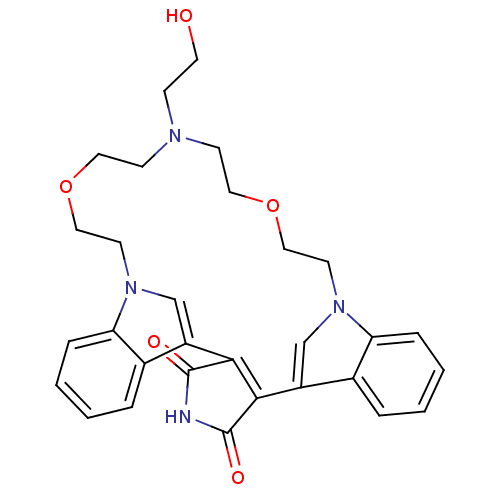
(20-(2-hydroxyethyl)-17,23-dioxa-4,14,20,26-tetraaz...)Show SMILES OCCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C30H32N4O5/c35-14-9-32-10-15-38-17-12-33-19-23(21-5-1-3-7-25(21)33)27-28(30(37)31-29(27)36)24-20-34(13-18-39-16-11-32)26-8-4-2-6-22(24)26/h1-8,19-20,35H,9-18H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132308

(20-(2-hydroxyethyl)-17,23-dioxa-4,14,20,26-tetraaz...)Show SMILES OCCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C30H32N4O5/c35-14-9-32-10-15-38-17-12-33-19-23(21-5-1-3-7-25(21)33)27-28(30(37)31-29(27)36)24-20-34(13-18-39-16-11-32)26-8-4-2-6-22(24)26/h1-8,19-20,35H,9-18H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132317

(17,23-dimethyl-20-oxa-4,14,17,23,26-pentaazahexacy...)Show SMILES CN1CCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:14| Show InChI InChI=1S/C30H33N5O3/c1-32-11-13-34-19-23(21-7-3-5-9-25(21)34)27-28(30(37)31-29(27)36)24-20-35(26-10-6-4-8-22(24)26)14-12-33(2)16-18-38-17-15-32/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132313
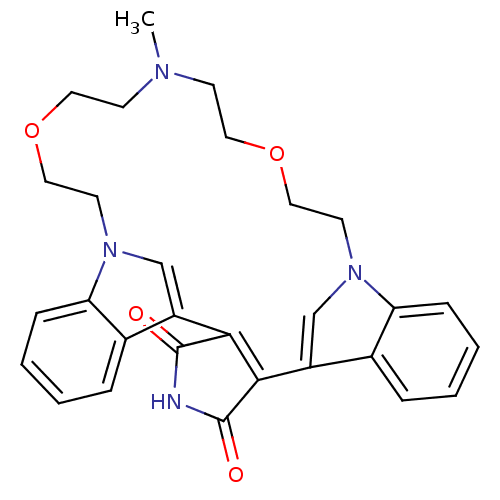
(20-methyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C29H30N4O4/c1-31-10-14-36-16-12-32-18-22(20-6-2-4-8-24(20)32)26-27(29(35)30-28(26)34)23-19-33(13-17-37-15-11-31)25-9-5-3-7-21(23)25/h2-9,18-19H,10-17H2,1H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132315

(17,20,23-trimethyl-4,14,17,20,23,26-hexaazahexacyc...)Show SMILES CN1CCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C31H36N6O2/c1-33-12-14-34(2)16-18-36-20-24(22-8-4-6-10-26(22)36)28-29(31(39)32-30(28)38)25-21-37(19-17-35(3)15-13-33)27-11-7-5-9-23(25)27/h4-11,20-21H,12-19H2,1-3H3,(H,32,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132318

(3-(1-{2-[2-(2-Dimethylamino-ethoxy)-ethoxy]-ethyl}...)Show SMILES CN1CCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCCOCC1)c1ccccc31)c1ccccc21 |t:8| Show InChI InChI=1S/C30H32N4O4/c1-32-11-6-12-33-19-23(21-7-2-4-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-5-3-8-22(24)26)14-16-38-18-17-37-15-13-32/h2-5,7-10,19-20H,6,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132318

(3-(1-{2-[2-(2-Dimethylamino-ethoxy)-ethoxy]-ethyl}...)Show SMILES CN1CCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCCOCC1)c1ccccc31)c1ccccc21 |t:8| Show InChI InChI=1S/C30H32N4O4/c1-32-11-6-12-33-19-23(21-7-2-4-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-5-3-8-22(24)26)14-16-38-18-17-37-15-13-32/h2-5,7-10,19-20H,6,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132311

(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132311

(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132318

(3-(1-{2-[2-(2-Dimethylamino-ethoxy)-ethoxy]-ethyl}...)Show SMILES CN1CCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCCOCC1)c1ccccc31)c1ccccc21 |t:8| Show InChI InChI=1S/C30H32N4O4/c1-32-11-6-12-33-19-23(21-7-2-4-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(26-10-5-3-8-22(24)26)14-16-38-18-17-37-15-13-32/h2-5,7-10,19-20H,6,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132313

(20-methyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C29H30N4O4/c1-31-10-14-36-16-12-32-18-22(20-6-2-4-8-24(20)32)26-27(29(35)30-28(26)34)23-19-33(13-17-37-15-11-31)25-9-5-3-7-21(23)25/h2-9,18-19H,10-17H2,1H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132308

(20-(2-hydroxyethyl)-17,23-dioxa-4,14,20,26-tetraaz...)Show SMILES OCCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C30H32N4O5/c35-14-9-32-10-15-38-17-12-33-19-23(21-5-1-3-7-25(21)33)27-28(30(37)31-29(27)36)24-20-34(13-18-39-16-11-32)26-8-4-2-6-22(24)26/h1-8,19-20,35H,9-18H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C gamma |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132308

(20-(2-hydroxyethyl)-17,23-dioxa-4,14,20,26-tetraaz...)Show SMILES OCCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:12| Show InChI InChI=1S/C30H32N4O5/c35-14-9-32-10-15-38-17-12-33-19-23(21-5-1-3-7-25(21)33)27-28(30(37)31-29(27)36)24-20-34(13-18-39-16-11-32)26-8-4-2-6-22(24)26/h1-8,19-20,35H,9-18H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50132313

(20-methyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C29H30N4O4/c1-31-10-14-36-16-12-32-18-22(20-6-2-4-8-24(20)32)26-27(29(35)30-28(26)34)23-19-33(13-17-37-15-11-31)25-9-5-3-7-21(23)25/h2-9,18-19H,10-17H2,1H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50132316

(17,26-dimethyl-20,23-dioxa-4,14,17,26,29-pentaazah...)Show SMILES CN1CCOCCOCCN(C)CCn2cc(C3=C(C(=O)NC3=O)c3cn(CC1)c1ccccc31)c1ccccc21 |t:17| Show InChI InChI=1S/C32H37N5O4/c1-34-11-13-36-21-25(23-7-3-5-9-27(23)36)29-30(32(39)33-31(29)38)26-22-37(28-10-6-4-8-24(26)28)14-12-35(2)16-18-41-20-19-40-17-15-34/h3-10,21-22H,11-20H2,1-2H3,(H,33,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50132310

(20-ethyl-17,23-dioxa-4,14,20,26-tetraazahexacyclo[...)Show SMILES CCN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCOCC1)c1ccccc31)c1ccccc21 |t:11| Show InChI InChI=1S/C30H32N4O4/c1-2-32-11-15-37-17-13-33-19-23(21-7-3-5-9-25(21)33)27-28(30(36)31-29(27)35)24-20-34(14-18-38-16-12-32)26-10-6-4-8-22(24)26/h3-10,19-20H,2,11-18H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50132311

(20,23-dimethyl-17-oxa-4,14,20,23,26-pentaazahexacy...)Show SMILES CN1CCOCCn2cc(C3=C(C(=O)NC3=O)c3cn(CCN(C)CC1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C30H33N5O3/c1-32-11-12-33(2)15-17-38-18-16-35-20-24(22-8-4-6-10-26(22)35)28-27(29(36)31-30(28)37)23-19-34(14-13-32)25-9-5-3-7-21(23)25/h3-10,19-20H,11-18H2,1-2H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 3049-53 (2003)
BindingDB Entry DOI: 10.7270/Q2DR2TVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data