 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50013006
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50013006 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
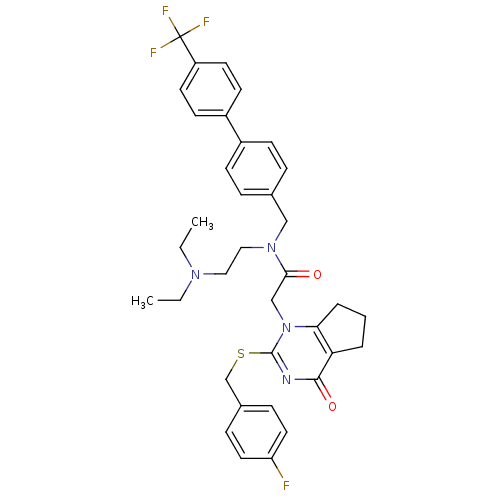
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
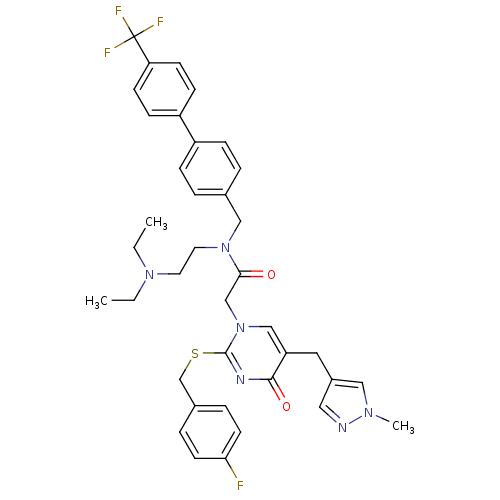
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125266
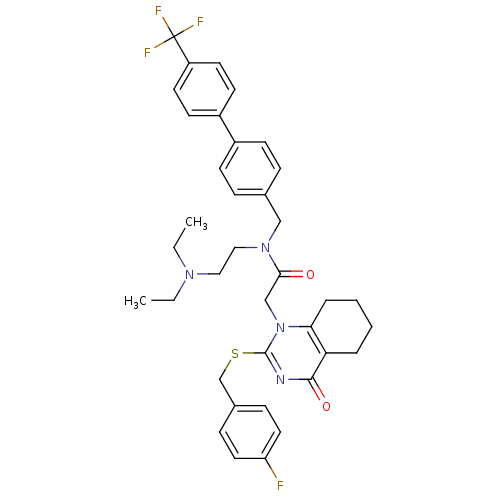
(CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C37H40F4N4O2S/c1-3-43(4-2)21-22-44(23-26-9-13-28(14-10-26)29-15-17-30(18-16-29)37(39,40)41)34(46)24-45-33-8-6-5-7-32(33)35(47)42-36(45)48-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 by mechanistic studies |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125267
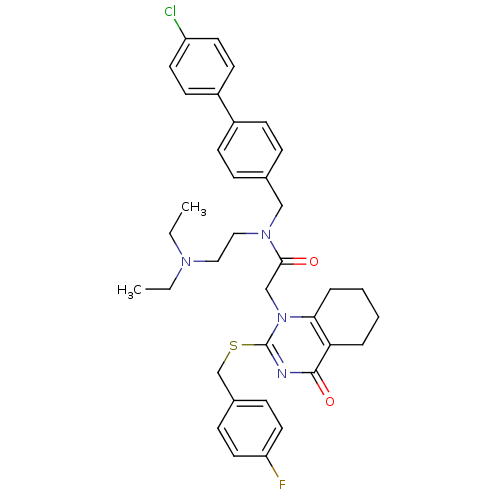
(CHEMBL10501 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C36H40ClFN4O2S/c1-3-40(4-2)21-22-41(23-26-9-13-28(14-10-26)29-15-17-30(37)18-16-29)34(43)24-42-33-8-6-5-7-32(33)35(44)39-36(42)45-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125272
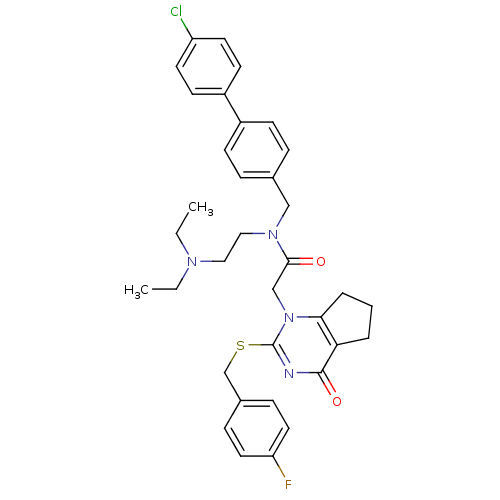
(CHEMBL10663 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38ClFN4O2S/c1-3-39(4-2)20-21-40(22-25-8-12-27(13-9-25)28-14-16-29(36)17-15-28)33(42)23-41-32-7-5-6-31(32)34(43)38-35(41)44-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125268
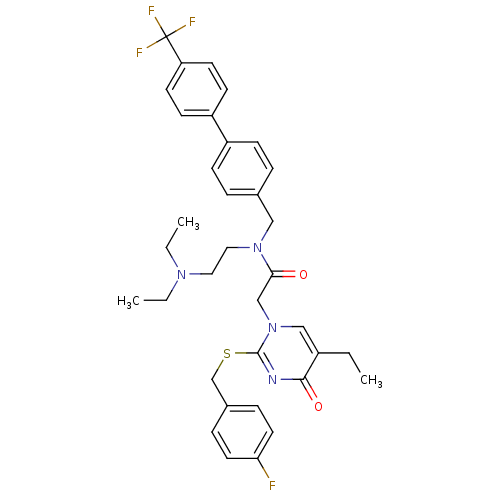
(CHEMBL10604 | N-(2-Diethylamino-ethyl)-2-[5-ethyl-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-4-27-22-43(34(40-33(27)45)46-24-26-9-17-31(36)18-10-26)23-32(44)42(20-19-41(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(16-14-29)35(37,38)39/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125270
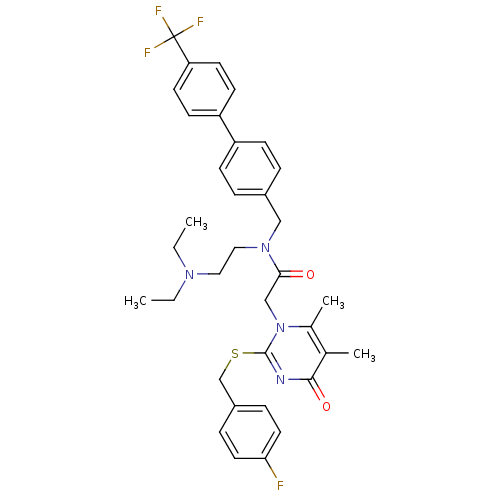
(CHEMBL10759 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)c(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-5-41(6-2)19-20-42(21-26-7-11-28(12-8-26)29-13-15-30(16-14-29)35(37,38)39)32(44)22-43-25(4)24(3)33(45)40-34(43)46-23-27-9-17-31(36)18-10-27/h7-18H,5-6,19-23H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125278
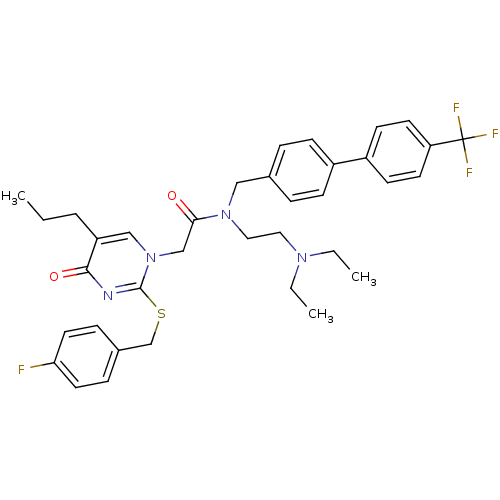
(CHEMBL274551 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCCc1cn(CC(=O)N(CCN(CC)CC)Cc2ccc(cc2)-c2ccc(cc2)C(F)(F)F)c(SCc2ccc(F)cc2)nc1=O Show InChI InChI=1S/C36H40F4N4O2S/c1-4-7-30-23-44(35(41-34(30)46)47-25-27-10-18-32(37)19-11-27)24-33(45)43(21-20-42(5-2)6-3)22-26-8-12-28(13-9-26)29-14-16-31(17-15-29)36(38,39)40/h8-19,23H,4-7,20-22,24-25H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125264
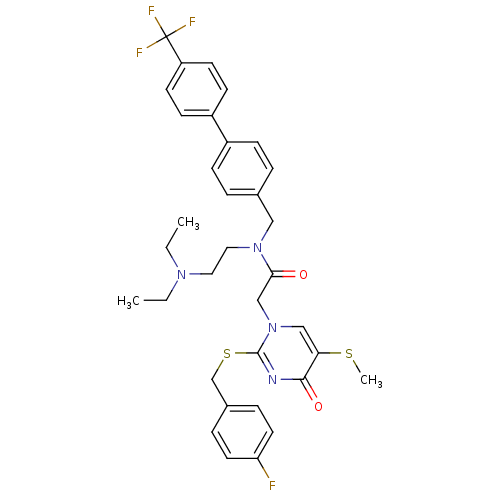
(CHEMBL10600 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(SC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S2/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(45-3)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125283
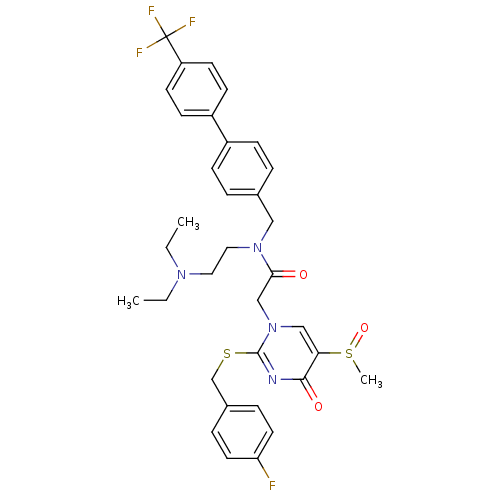
(CHEMBL275128 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(S(C)=O)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O3S2/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(47(3)45)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125282

(CHEMBL10352 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CCNS(C)(=O)=O)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H41F4N5O4S2/c1-4-43(5-2)20-21-44(22-26-6-10-28(11-7-26)29-12-14-31(15-13-29)36(38,39)40)33(46)24-45-23-30(18-19-41-51(3,48)49)34(47)42-35(45)50-25-27-8-16-32(37)17-9-27/h6-17,23,41H,4-5,18-22,24-25H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125276
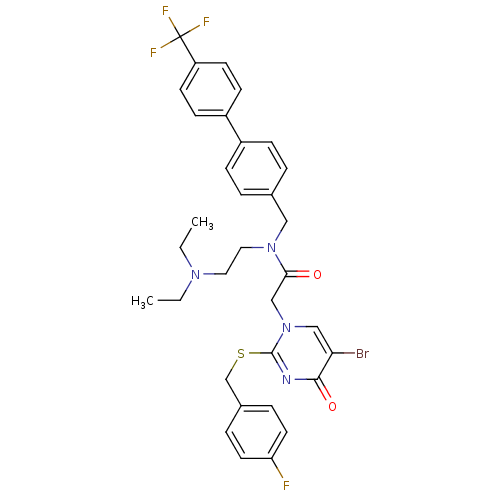
(2-[5-Bromo-2-(4-fluoro-benzylsulfanyl)-4-oxo-4H-py...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Br)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H33BrF4N4O2S/c1-3-40(4-2)17-18-41(19-23-5-9-25(10-6-23)26-11-13-27(14-12-26)33(36,37)38)30(43)21-42-20-29(34)31(44)39-32(42)45-22-24-7-15-28(35)16-8-24/h5-16,20H,3-4,17-19,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125263
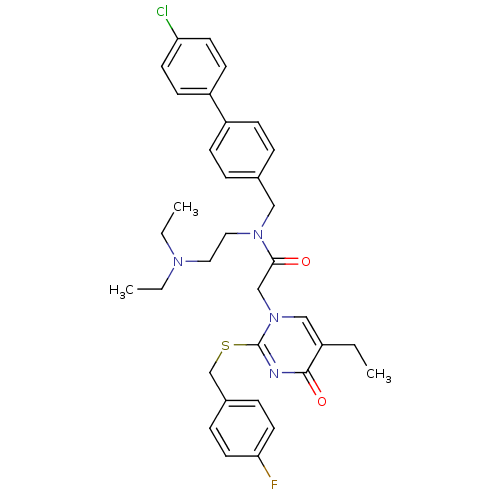
(CHEMBL273503 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H38ClFN4O2S/c1-4-27-22-40(34(37-33(27)42)43-24-26-9-17-31(36)18-10-26)23-32(41)39(20-19-38(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(35)16-14-29/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125262
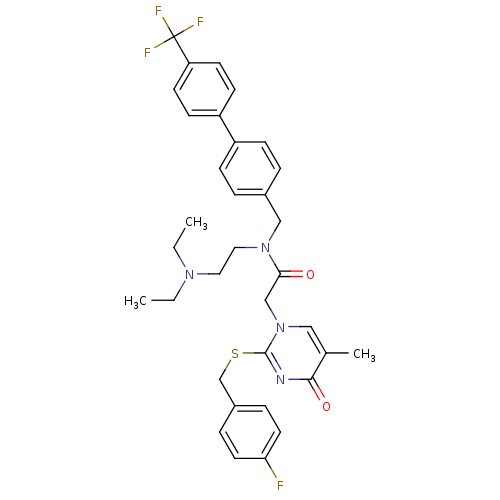
(CHEMBL10532 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S/c1-4-40(5-2)18-19-41(21-25-6-10-27(11-7-25)28-12-14-29(15-13-28)34(36,37)38)31(43)22-42-20-24(3)32(44)39-33(42)45-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125280

(CHEMBL165840 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CO)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O3S/c1-3-40(4-2)17-18-41(19-24-5-9-26(10-6-24)27-11-13-29(14-12-27)34(36,37)38)31(44)21-42-20-28(22-43)32(45)39-33(42)46-23-25-7-15-30(35)16-8-25/h5-16,20,43H,3-4,17-19,21-23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125279
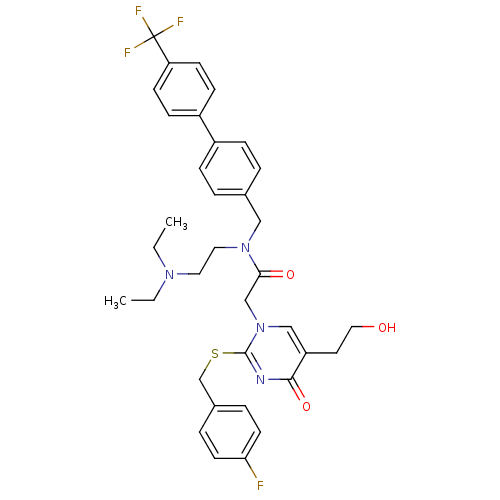
(CHEMBL10502 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CCO)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O3S/c1-3-41(4-2)18-19-42(21-25-5-9-27(10-6-25)28-11-13-30(14-12-28)35(37,38)39)32(45)23-43-22-29(17-20-44)33(46)40-34(43)47-24-26-7-15-31(36)16-8-26/h5-16,22,44H,3-4,17-21,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125277
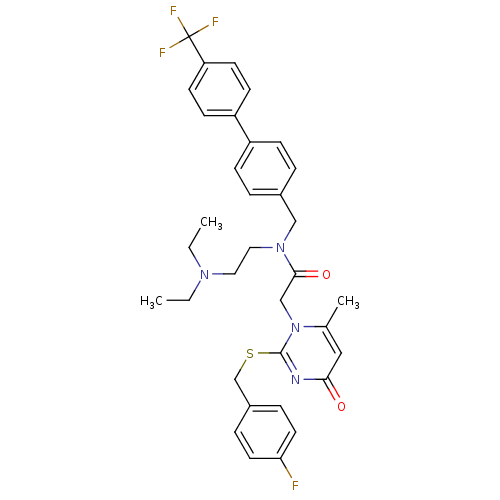
(CHEMBL268764 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)cc(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S/c1-4-40(5-2)18-19-41(21-25-6-10-27(11-7-25)28-12-14-29(15-13-28)34(36,37)38)32(44)22-42-24(3)20-31(43)39-33(42)45-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125274
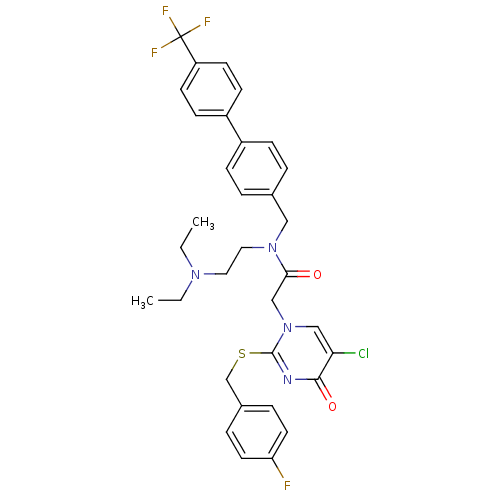
(2-[5-Chloro-2-(4-fluoro-benzylsulfanyl)-4-oxo-4H-p...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cl)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H33ClF4N4O2S/c1-3-40(4-2)17-18-41(19-23-5-9-25(10-6-23)26-11-13-27(14-12-26)33(36,37)38)30(43)21-42-20-29(34)31(44)39-32(42)45-22-24-7-15-28(35)16-8-24/h5-16,20H,3-4,17-19,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Lp-PLA2 in whole human plasma |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125275
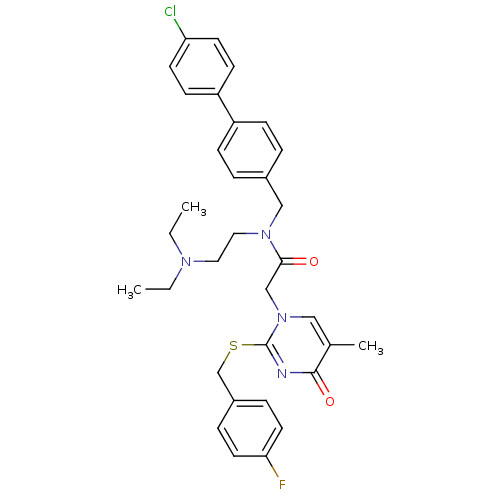
(CHEMBL10440 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H36ClFN4O2S/c1-4-37(5-2)18-19-38(21-25-6-10-27(11-7-25)28-12-14-29(34)15-13-28)31(40)22-39-20-24(3)32(41)36-33(39)42-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125273
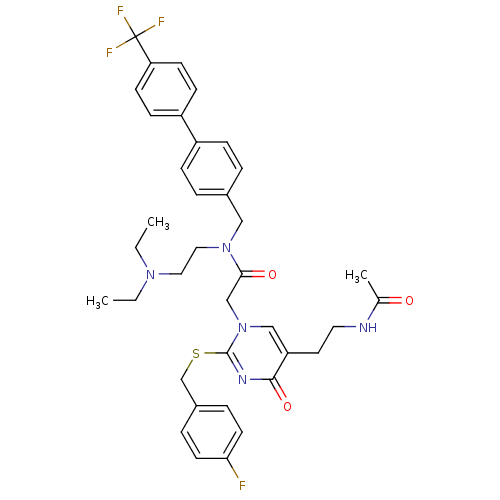
(2-[5-(2-Acetylamino-ethyl)-2-(4-fluoro-benzylsulfa...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CCNC(C)=O)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C37H41F4N5O3S/c1-4-44(5-2)20-21-45(22-27-6-10-29(11-7-27)30-12-14-32(15-13-30)37(39,40)41)34(48)24-46-23-31(18-19-42-26(3)47)35(49)43-36(46)50-25-28-8-16-33(38)17-9-28/h6-17,23H,4-5,18-22,24-25H2,1-3H3,(H,42,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125269
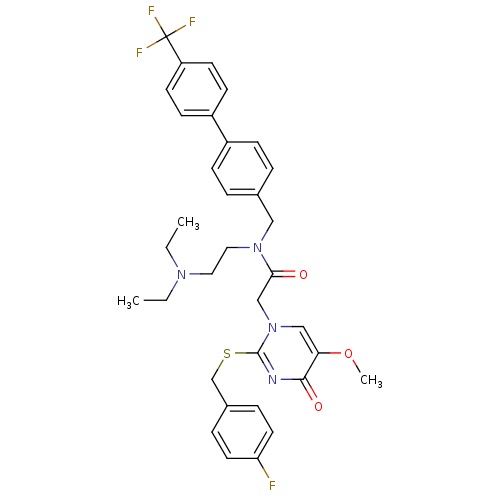
(CHEMBL10612 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(OC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O3S/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(45-3)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125281
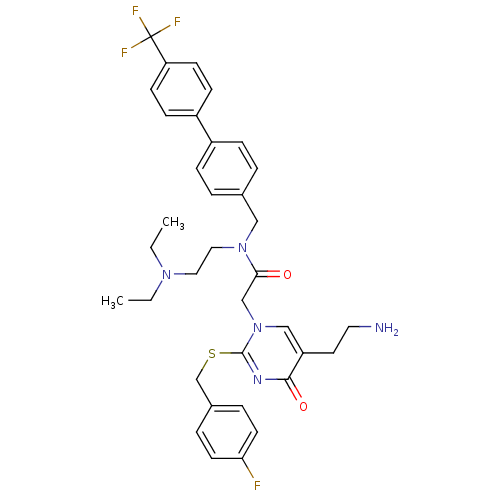
(2-[5-(2-Amino-ethyl)-2-(4-fluoro-benzylsulfanyl)-4...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CCN)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H39F4N5O2S/c1-3-42(4-2)19-20-43(21-25-5-9-27(10-6-25)28-11-13-30(14-12-28)35(37,38)39)32(45)23-44-22-29(17-18-40)33(46)41-34(44)47-24-26-7-15-31(36)16-8-26/h5-16,22H,3-4,17-21,23-24,40H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125271
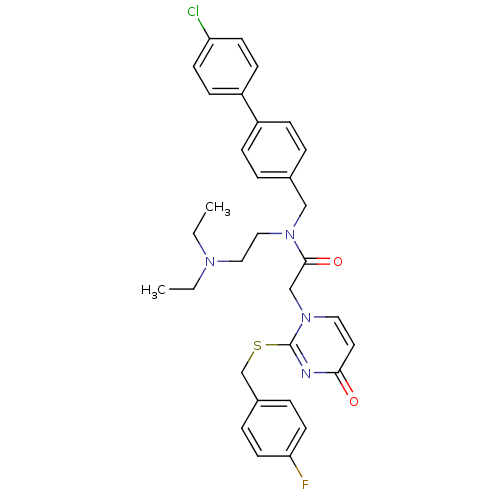
(CHEMBL10864 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1ccc(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H34ClFN4O2S/c1-3-36(4-2)19-20-37(21-24-5-9-26(10-6-24)27-11-13-28(33)14-12-27)31(40)22-38-18-17-30(39)35-32(38)41-23-25-7-15-29(34)16-8-25/h5-18H,3-4,19-23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125267

(CHEMBL10501 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C36H40ClFN4O2S/c1-3-40(4-2)21-22-41(23-26-9-13-28(14-10-26)29-15-17-30(37)18-16-29)34(43)24-42-33-8-6-5-7-32(33)35(44)39-36(42)45-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125278

(CHEMBL274551 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCCc1cn(CC(=O)N(CCN(CC)CC)Cc2ccc(cc2)-c2ccc(cc2)C(F)(F)F)c(SCc2ccc(F)cc2)nc1=O Show InChI InChI=1S/C36H40F4N4O2S/c1-4-7-30-23-44(35(41-34(30)46)47-25-27-10-18-32(37)19-11-27)24-33(45)43(21-20-42(5-2)6-3)22-26-8-12-28(13-9-26)29-14-16-31(17-15-29)36(38,39)40/h8-19,23H,4-7,20-22,24-25H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125267

(CHEMBL10501 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C36H40ClFN4O2S/c1-3-40(4-2)21-22-41(23-26-9-13-28(14-10-26)29-15-17-30(37)18-16-29)34(43)24-42-33-8-6-5-7-32(33)35(44)39-36(42)45-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125263

(CHEMBL273503 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H38ClFN4O2S/c1-4-27-22-40(34(37-33(27)42)43-24-26-9-17-31(36)18-10-26)23-32(41)39(20-19-38(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(35)16-14-29/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125266

(CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C37H40F4N4O2S/c1-3-43(4-2)21-22-44(23-26-9-13-28(14-10-26)29-15-17-30(18-16-29)37(39,40)41)34(46)24-45-33-8-6-5-7-32(33)35(47)42-36(45)48-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125266

(CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C37H40F4N4O2S/c1-3-43(4-2)21-22-44(23-26-9-13-28(14-10-26)29-15-17-30(18-16-29)37(39,40)41)34(46)24-45-33-8-6-5-7-32(33)35(47)42-36(45)48-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125268

(CHEMBL10604 | N-(2-Diethylamino-ethyl)-2-[5-ethyl-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-4-27-22-43(34(40-33(27)45)46-24-26-9-17-31(36)18-10-26)23-32(44)42(20-19-41(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(16-14-29)35(37,38)39/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125275

(CHEMBL10440 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H36ClFN4O2S/c1-4-37(5-2)18-19-38(21-25-6-10-27(11-7-25)28-12-14-29(34)15-13-28)31(40)22-39-20-24(3)32(41)36-33(39)42-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125263

(CHEMBL273503 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H38ClFN4O2S/c1-4-27-22-40(34(37-33(27)42)43-24-26-9-17-31(36)18-10-26)23-32(41)39(20-19-38(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(35)16-14-29/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125275

(CHEMBL10440 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H36ClFN4O2S/c1-4-37(5-2)18-19-38(21-25-6-10-27(11-7-25)28-12-14-29(34)15-13-28)31(40)22-39-20-24(3)32(41)36-33(39)42-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125264

(CHEMBL10600 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(SC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S2/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(45-3)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125262

(CHEMBL10532 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S/c1-4-40(5-2)18-19-41(21-25-6-10-27(11-7-25)28-12-14-29(15-13-28)34(36,37)38)31(43)22-42-20-24(3)32(44)39-33(42)45-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125272

(CHEMBL10663 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38ClFN4O2S/c1-3-39(4-2)20-21-40(22-25-8-12-27(13-9-25)28-14-16-29(36)17-15-28)33(42)23-41-32-7-5-6-31(32)34(43)38-35(41)44-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125277

(CHEMBL268764 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)cc(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S/c1-4-40(5-2)18-19-41(21-25-6-10-27(11-7-25)28-12-14-29(15-13-28)34(36,37)38)32(44)22-42-24(3)20-31(43)39-33(42)45-23-26-8-16-30(35)17-9-26/h6-17,20H,4-5,18-19,21-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125270

(CHEMBL10759 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)c(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-5-41(6-2)19-20-42(21-26-7-11-28(12-8-26)29-13-15-30(16-14-29)35(37,38)39)32(44)22-43-25(4)24(3)33(45)40-34(43)46-23-27-9-17-31(36)18-10-27/h7-18H,5-6,19-23H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125278

(CHEMBL274551 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCCc1cn(CC(=O)N(CCN(CC)CC)Cc2ccc(cc2)-c2ccc(cc2)C(F)(F)F)c(SCc2ccc(F)cc2)nc1=O Show InChI InChI=1S/C36H40F4N4O2S/c1-4-7-30-23-44(35(41-34(30)46)47-25-27-10-18-32(37)19-11-27)24-33(45)43(21-20-42(5-2)6-3)22-26-8-12-28(13-9-26)29-14-16-31(17-15-29)36(38,39)40/h8-19,23H,4-7,20-22,24-25H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125270

(CHEMBL10759 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)c(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-5-41(6-2)19-20-42(21-26-7-11-28(12-8-26)29-13-15-30(16-14-29)35(37,38)39)32(44)22-43-25(4)24(3)33(45)40-34(43)46-23-27-9-17-31(36)18-10-27/h7-18H,5-6,19-23H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125268

(CHEMBL10604 | N-(2-Diethylamino-ethyl)-2-[5-ethyl-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-4-27-22-43(34(40-33(27)45)46-24-26-9-17-31(36)18-10-26)23-32(44)42(20-19-41(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(16-14-29)35(37,38)39/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125272

(CHEMBL10663 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38ClFN4O2S/c1-3-39(4-2)20-21-40(22-25-8-12-27(13-9-25)28-14-16-29(36)17-15-28)33(42)23-41-32-7-5-6-31(32)34(43)38-35(41)44-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125274

(2-[5-Chloro-2-(4-fluoro-benzylsulfanyl)-4-oxo-4H-p...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cl)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H33ClF4N4O2S/c1-3-40(4-2)17-18-41(19-23-5-9-25(10-6-23)26-11-13-27(14-12-26)33(36,37)38)30(43)21-42-20-29(34)31(44)39-32(42)45-22-24-7-15-28(35)16-8-24/h5-16,20H,3-4,17-19,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cytochrome P450 2D6 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50125276

(2-[5-Bromo-2-(4-fluoro-benzylsulfanyl)-4-oxo-4H-py...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Br)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H33BrF4N4O2S/c1-3-40(4-2)17-18-41(19-23-5-9-25(10-6-23)26-11-13-27(14-12-26)33(36,37)38)30(43)21-42-20-29(34)31(44)39-32(42)45-22-24-7-15-28(35)16-8-24/h5-16,20H,3-4,17-19,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP450 3A4 isozyme |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data