

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126462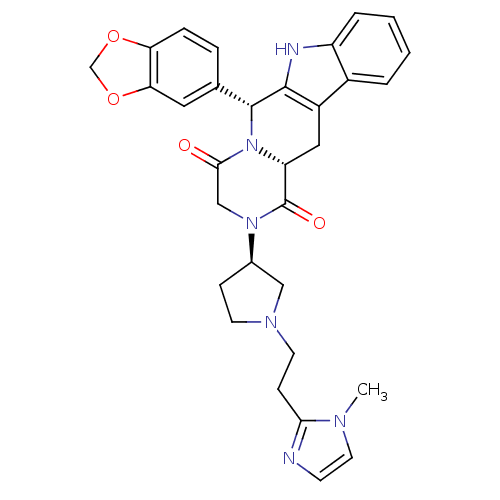 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126467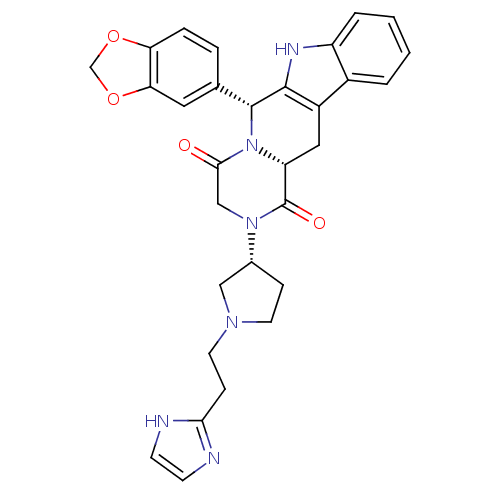 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126452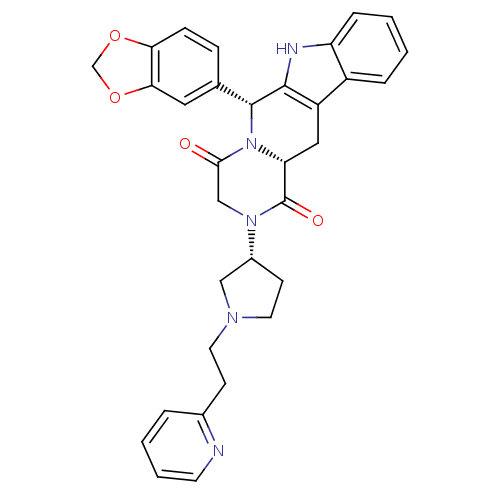 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126455 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126464 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126452 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126458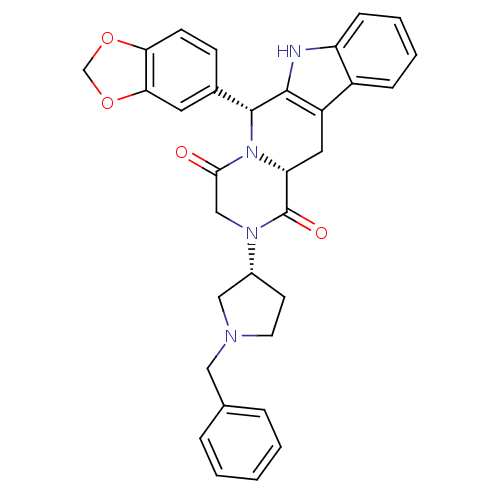 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-benzyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126455 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126449 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126451 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126467 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1H-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126453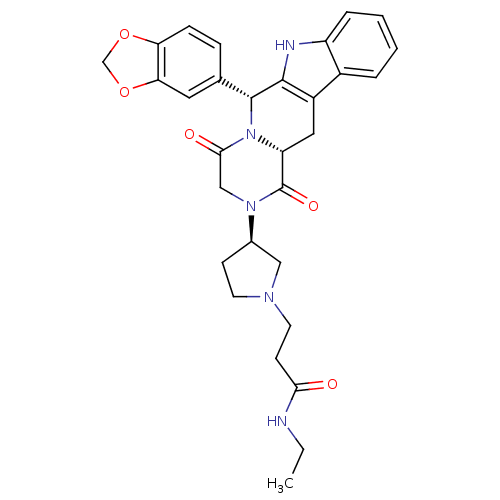 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126448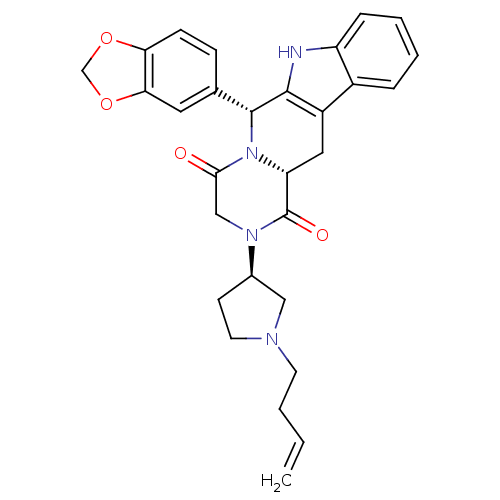 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-but-3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126456 ((6R,12aR)-2-[(R)-1-(3-Azetidin-1-yl-3-oxo-propyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126464 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyra...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126453 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126449 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126456 ((6R,12aR)-2-[(R)-1-(3-Azetidin-1-yl-3-oxo-propyl)-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126458 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-benzyl-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126463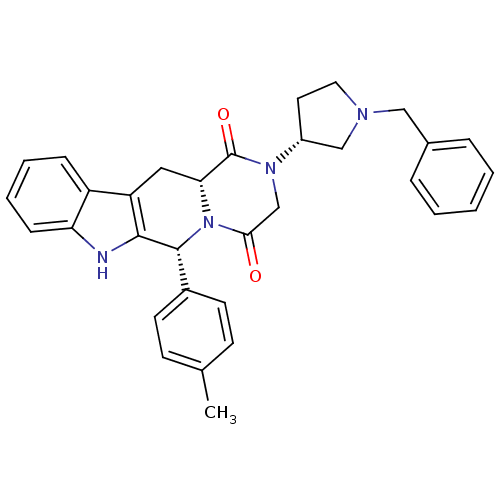 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-p-tol...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126451 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(4-me...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126457 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126470 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| O43924/P16499/P18545/P35913/P51160/Q13956 (Homo sapiens (Human)) | BDBM50126469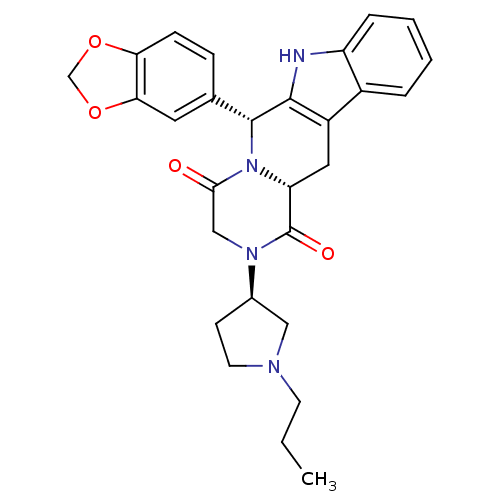 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-propyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 6 (PDE6) obtained from canine or bovine retina | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126461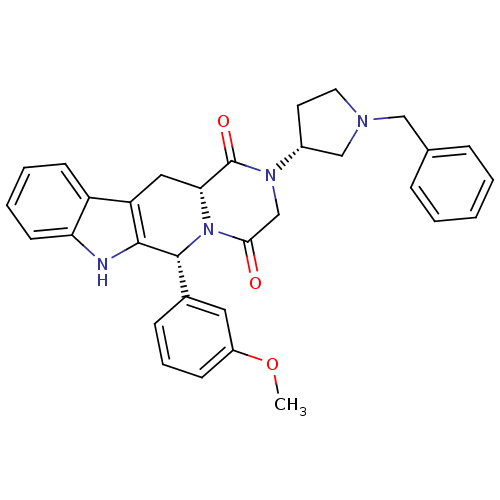 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(3-me...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126461 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(3-me...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126468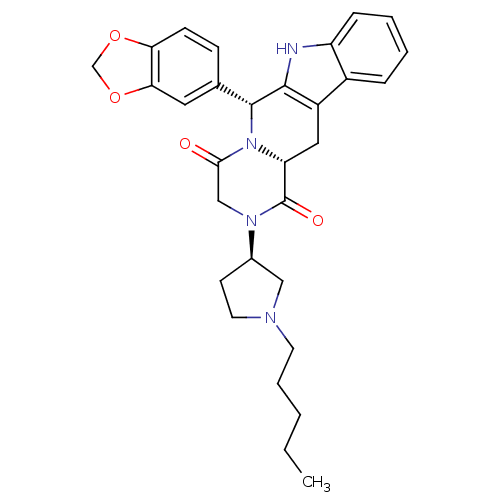 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-hexyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126463 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-p-tol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126461 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126461 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126466 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126447 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((S)-1-benzyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126459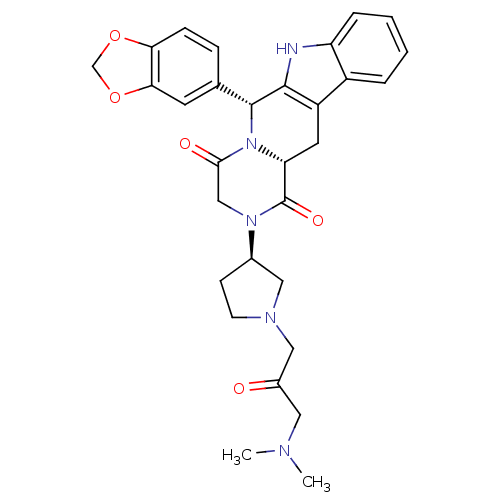 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(3-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126457 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(3-meth...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126473 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-(1-benzyl-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126468 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-hexyl-p...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126471 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(6-ox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126466 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-cycl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126459 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(3-dime...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126470 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126460 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-(2-dimethylami...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126465 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-(2-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126469 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126472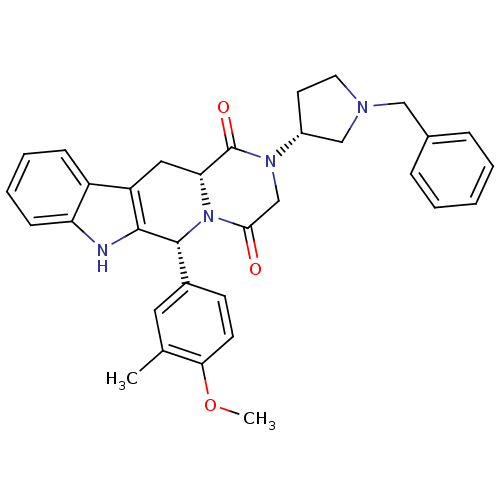 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(4-me...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126447 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((S)-1-benzyl-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |