

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006906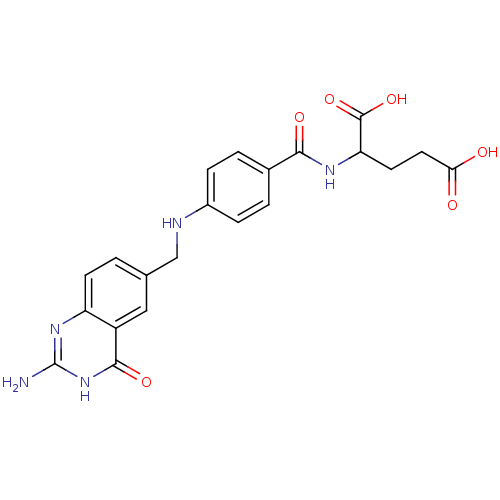 (2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50017876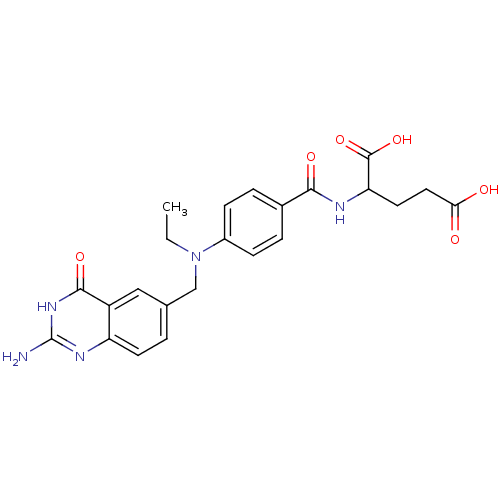 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006907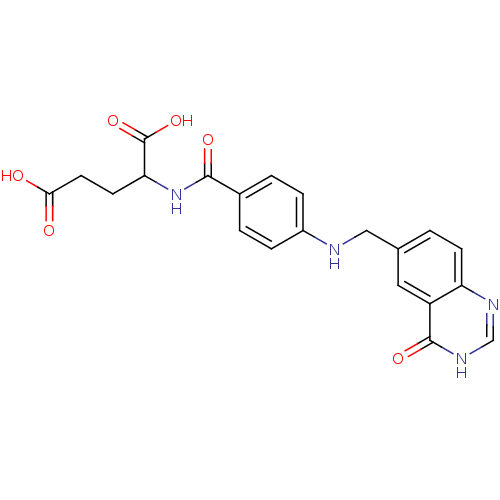 (2-{4-[(4-Oxo-3,4-dihydro-quinazolin-6-ylmethyl)-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50017875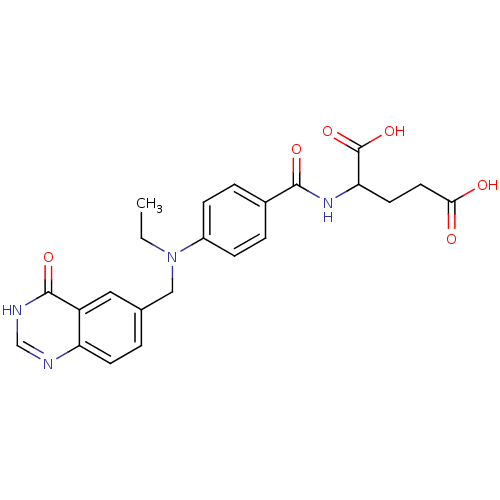 (2-{4-[Ethyl-(4-oxo-3,4-dihydro-quinazolin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase(DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50011243 (2-{4-[(4-Hydroxy-quinazolin-6-ylmethyl)-prop-2-yny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of partially purified rat liver Dihydrofolate reductase (DHFR) enzyme. | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50017876 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50011243 (2-{4-[(4-Hydroxy-quinazolin-6-ylmethyl)-prop-2-yny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50017873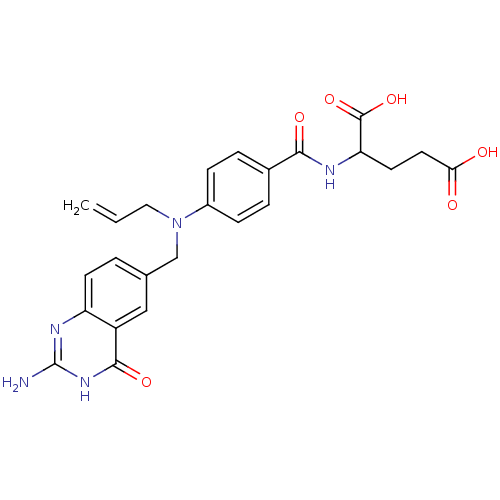 (2-{4-[Allyl-(2-amino-4-oxo-3,4-dihydro-quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50017872 (2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50017875 (2-{4-[Ethyl-(4-oxo-3,4-dihydro-quinazolin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50017877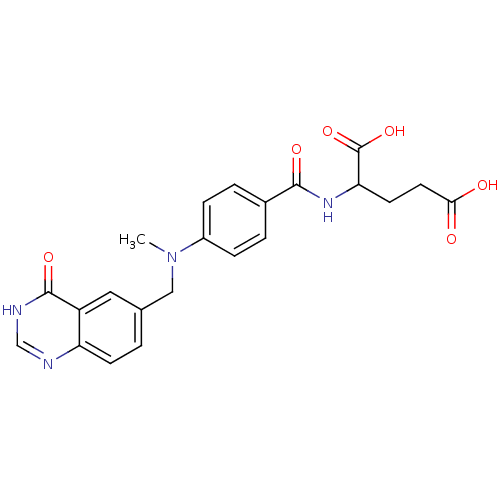 (2-{4-[Methyl-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50017874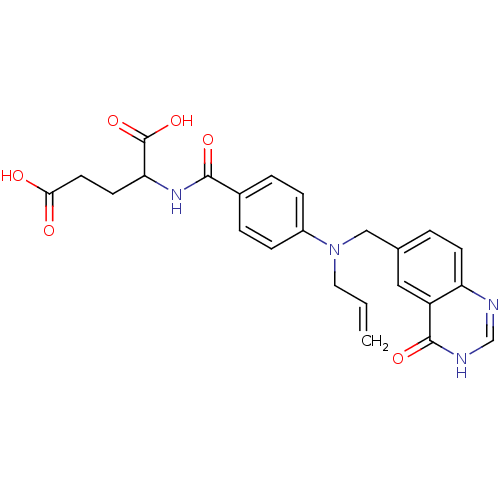 (2-{4-[Allyl-(4-oxo-3,4-dihydro-quinazolin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50006906 (2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 uM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50006907 (2-{4-[(4-Oxo-3,4-dihydro-quinazolin-6-ylmethyl)-am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory concentration of compound to inhibit Thymidylate synthase (TS) in L1210 cells at conc. of 200 microM | J Med Chem 32: 847-52 (1989) BindingDB Entry DOI: 10.7270/Q2BP01SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||