

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138479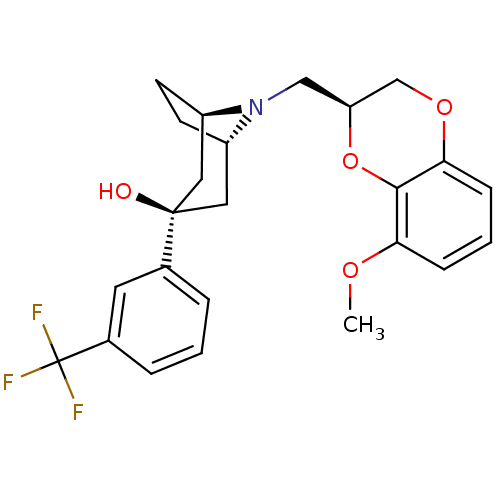 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138478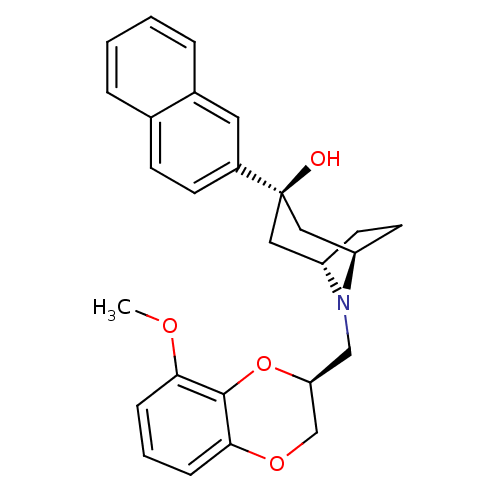 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138483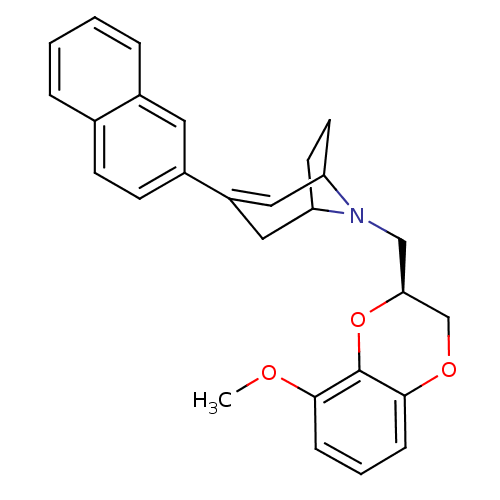 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138480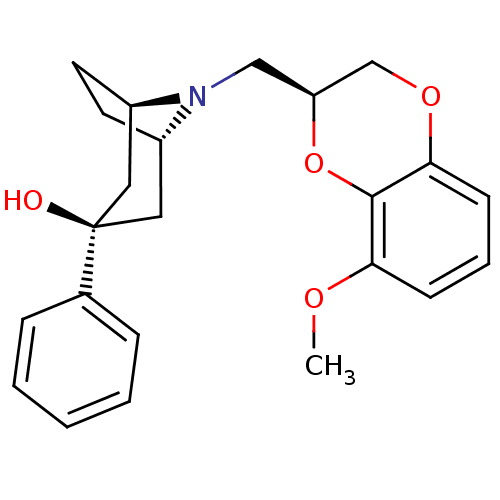 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138474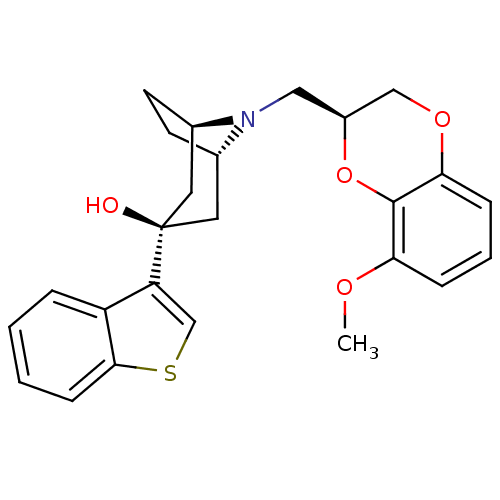 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138485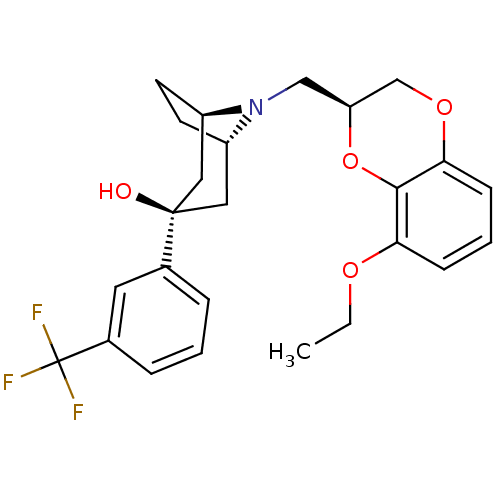 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138470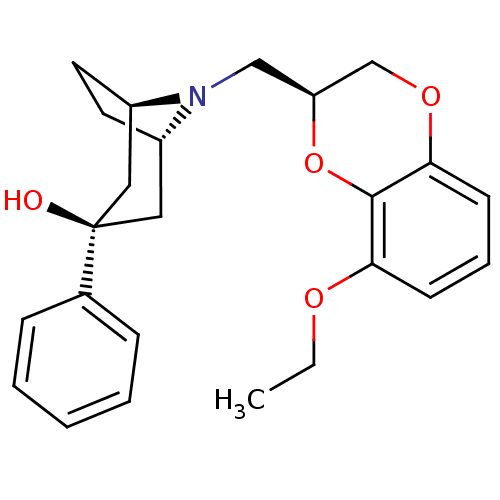 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138471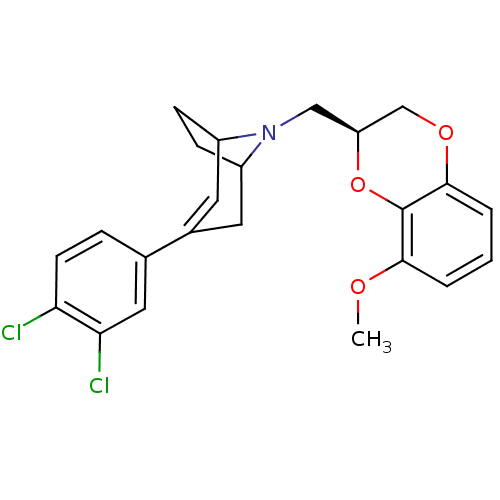 (3-(3,4-Dichloro-phenyl)-8-((S)-8-methoxy-2,3-dihyd...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138473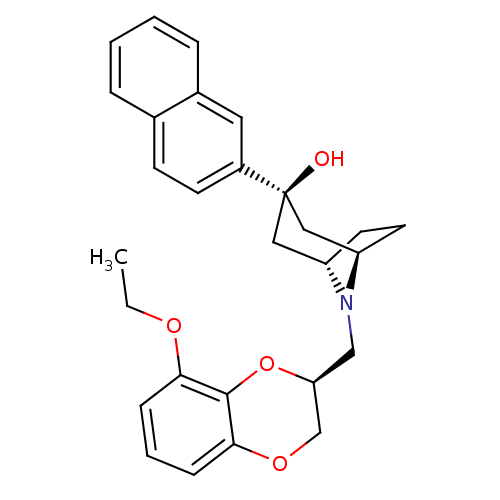 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138476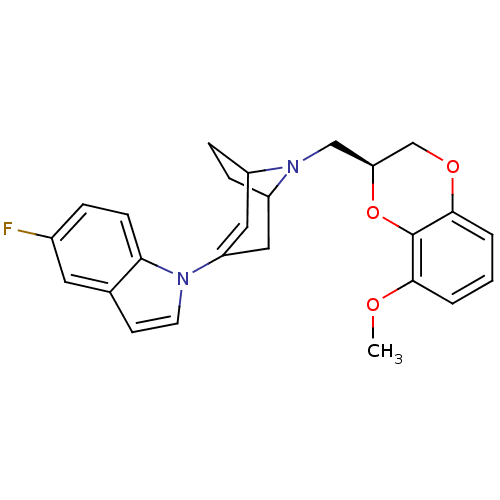 (5-Fluoro-1-[8-((S)-8-methoxy-2,3-dihydro-benzo[1,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138479 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138485 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138472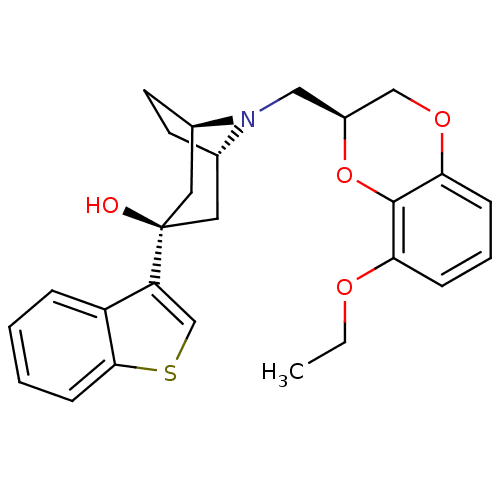 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138475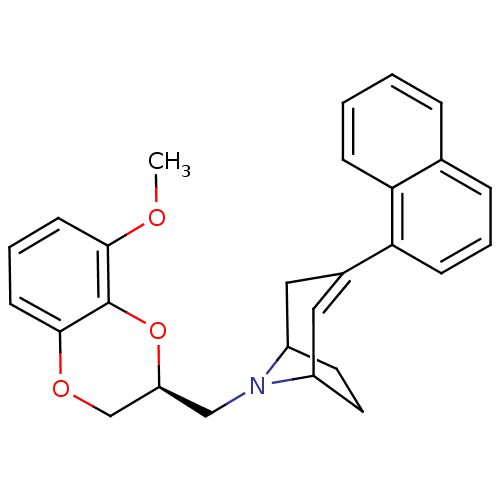 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138470 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138477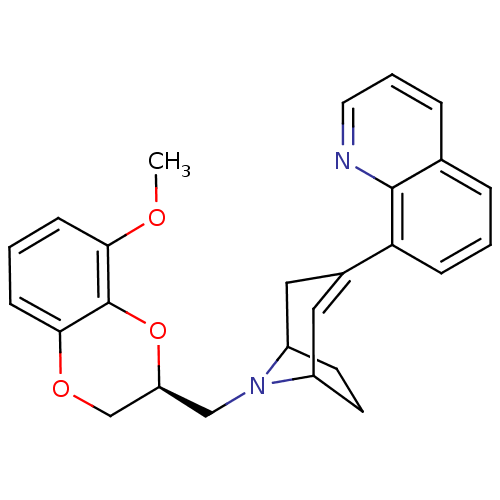 (8-[8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138484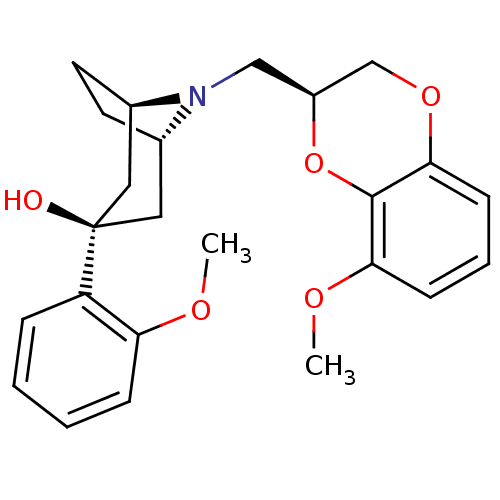 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138478 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by RB5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138481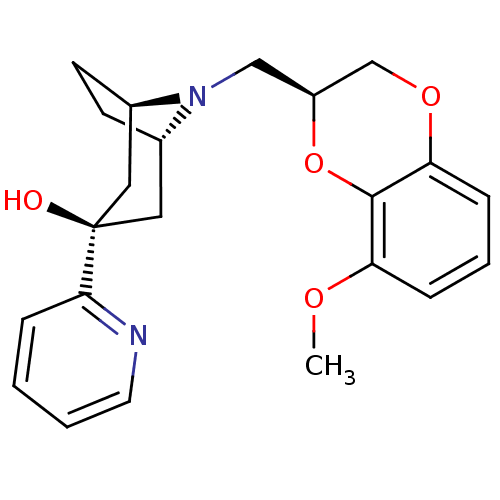 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138477 (8-[8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138482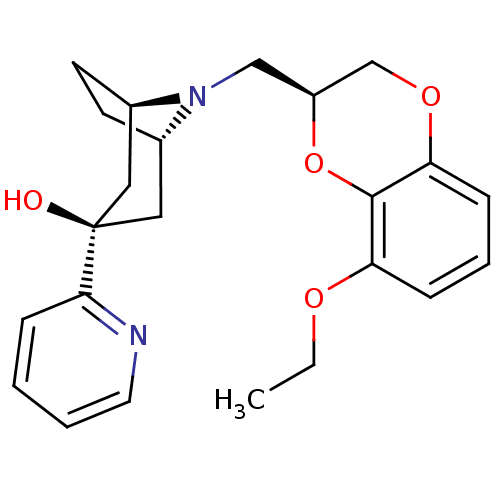 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138476 (5-Fluoro-1-[8-((S)-8-methoxy-2,3-dihydro-benzo[1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by [35S]-GTP-gammaS, stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138473 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138474 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by [35S]-GTP-gammaS, stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138472 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-ethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by [35S]-GTP-gammaS, stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138476 (5-Fluoro-1-[8-((S)-8-methoxy-2,3-dihydro-benzo[1,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138482 ((1R,3R,5S)-8-((S)-8-Ethoxy-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138483 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha-1 adrenergic receptor | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138477 (8-[8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138475 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50138471 (3-(3,4-Dichloro-phenyl)-8-((S)-8-methoxy-2,3-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138478 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by forskloin stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138480 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138478 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138480 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138474 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by [35S]-GTP-gammaS, stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138477 (8-[8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138474 ((1R,3R,5S)-3-Benzo[b]thiophen-3-yl-8-((S)-8-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by forskloin stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138476 (5-Fluoro-1-[8-((S)-8-methoxy-2,3-dihydro-benzo[1,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by forskloin stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138471 (3-(3,4-Dichloro-phenyl)-8-((S)-8-methoxy-2,3-dihyd...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138483 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 767 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by forskloin stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50138475 (8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]dioxin-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 951 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity of compound against serotonin transport by HC5-5-HT transporter | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50138481 ((1R,3R,5S)-8-((S)-8-Methoxy-2,3-dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonsitic activity of compound towards 5-hydroxytryptamine 1A receptor was evaluated by [35S]-GTP-gammaS, stimulated cAMP assay | Bioorg Med Chem Lett 14: 515-8 (2003) BindingDB Entry DOI: 10.7270/Q247499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||