

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028605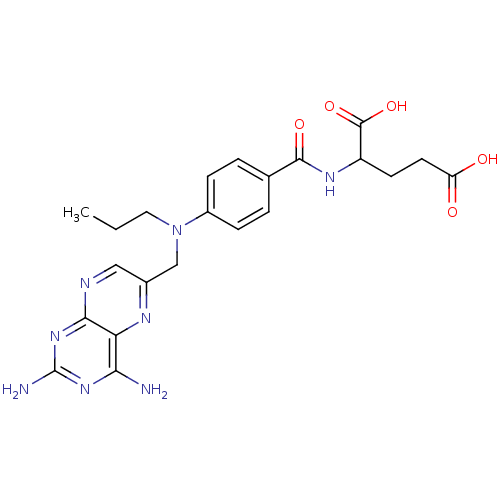 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-propyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028603 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-prop-2-yny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028604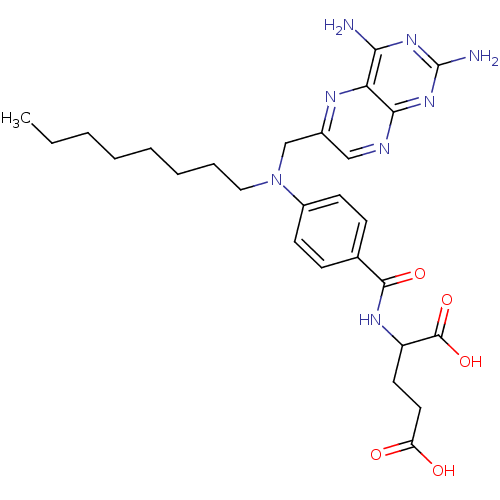 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-octyl-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50028603 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-prop-2-yny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50028604 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-octyl-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028603 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-prop-2-yny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of activity against Lactobacillus casei enzyme thymidylate synthase | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of activity against Lactobacillus casei enzyme thymidylate synthase | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028604 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-octyl-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of activity against Lactobacillus casei enzyme thymidylate synthase | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||