 Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50035934
Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50035934 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM10044
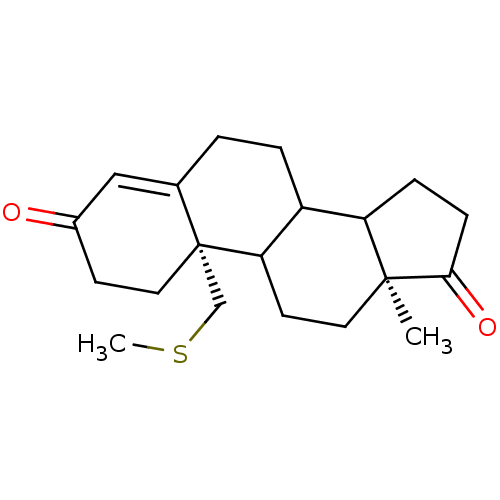
((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...)Show SMILES CSC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:8| Show InChI InChI=1S/C20H28O2S/c1-19-9-8-17-15(16(19)5-6-18(19)22)4-3-13-11-14(21)7-10-20(13,17)12-23-2/h11,15-17H,3-10,12H2,1-2H3/t15?,16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611
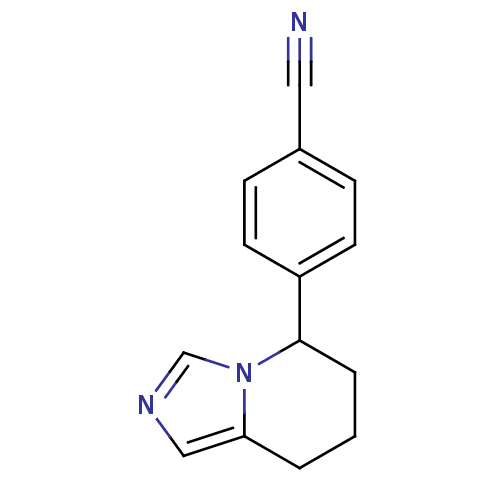
(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Competitive inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50368174

(CHEMBL1203762)Show InChI InChI=1S/C13H11N3/c14-7-10-1-3-11(4-2-10)13-6-5-12-8-15-9-16(12)13/h1-4,8-9,13H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50368173
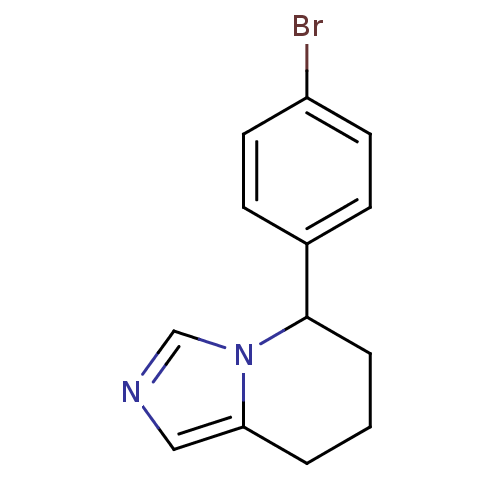
(CHEMBL1203766)Show InChI InChI=1S/C13H13BrN2/c14-11-6-4-10(5-7-11)13-3-1-2-12-8-15-9-16(12)13/h4-9,13H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008725

(4-(6,7,8,9-Tetrahydro-5H-imidazo[1,5-a]azepin-5-yl...)Show InChI InChI=1S/C15H15N3/c16-9-12-5-7-13(8-6-12)15-4-2-1-3-14-10-17-11-18(14)15/h5-8,10-11,15H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008726

(CHEMBL348913 | [4-(5,6,7,8-Tetrahydro-imidazo[1,5-...)Show InChI InChI=1S/C14H16N2O/c17-9-11-4-6-12(7-5-11)14-3-1-2-13-8-15-10-16(13)14/h4-8,10,14,17H,1-3,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008729
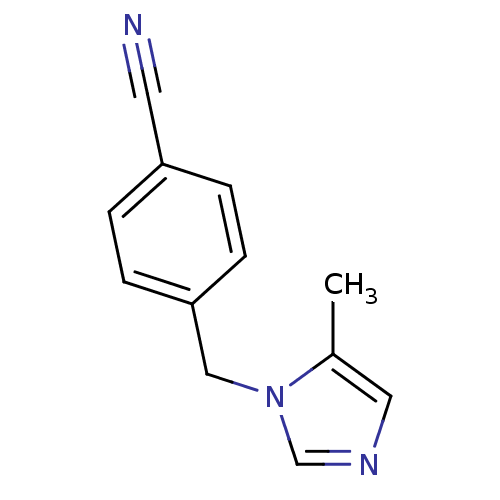
(1-(4-Cyanobenzyl)-5-methyl-1H-imidazole | 4-(5-Met...)Show InChI InChI=1S/C12H11N3/c1-10-7-14-9-15(10)8-12-4-2-11(6-13)3-5-12/h2-5,7,9H,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008733
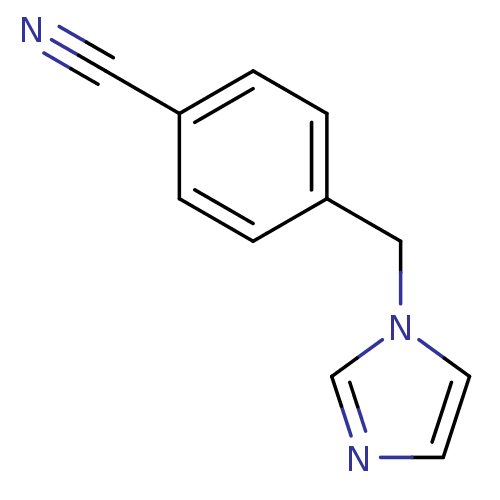
(1-(4-Cyanobenzyl)-1H-imidazole | 4-((1H-imidazol-1...)Show InChI InChI=1S/C11H9N3/c12-7-10-1-3-11(4-2-10)8-14-6-5-13-9-14/h1-6,9H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50368172
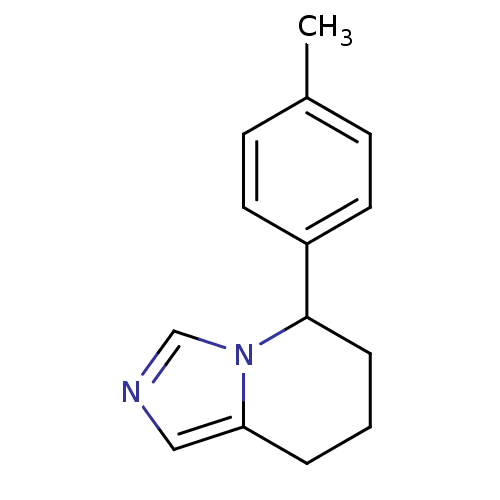
(CHEMBL1203757)Show InChI InChI=1S/C14H16N2/c1-11-5-7-12(8-6-11)14-4-2-3-13-9-15-10-16(13)14/h5-10,14H,2-4H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50368175

(CHEMBL1203760)Show InChI InChI=1S/C16H18N2O2/c1-2-20-16(19)13-8-6-12(7-9-13)15-5-3-4-14-10-17-11-18(14)15/h6-11,15H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008724
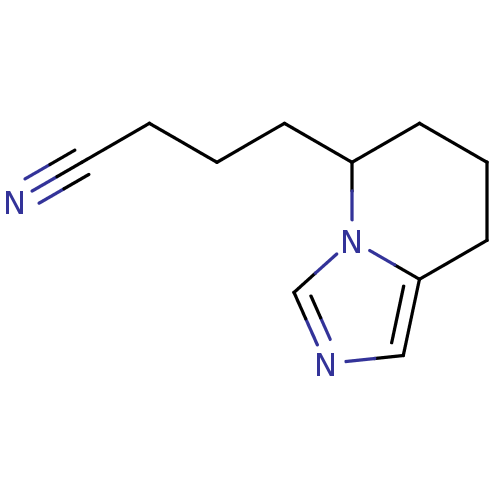
(4-(5,6,7,8-Tetrahydro-imidazo[1,5-a]pyridin-5-yl)-...)Show InChI InChI=1S/C11H15N3/c12-7-2-1-4-10-5-3-6-11-8-13-9-14(10)11/h8-10H,1-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Rattus norvegicus) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ACTH-stimulated aldosterone production in rat adrenal tissue |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50008730

(4-(5,6,7,8-Tetrahydro-imidazo[1,5-a]pyridin-5-yl)-...)Show InChI InChI=1S/C14H14N2O2/c17-14(18)11-6-4-10(5-7-11)13-3-1-2-12-8-15-9-16(12)13/h4-9,13H,1-3H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of progesterone production in hamster ovarian tissue |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Rattus norvegicus) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ACTH-stimulated corticosterone production in rat adrenal tissue |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data