 Found 70 hits Enz. Inhib. hit(s) with all data for entry = 50004888
Found 70 hits Enz. Inhib. hit(s) with all data for entry = 50004888 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029613
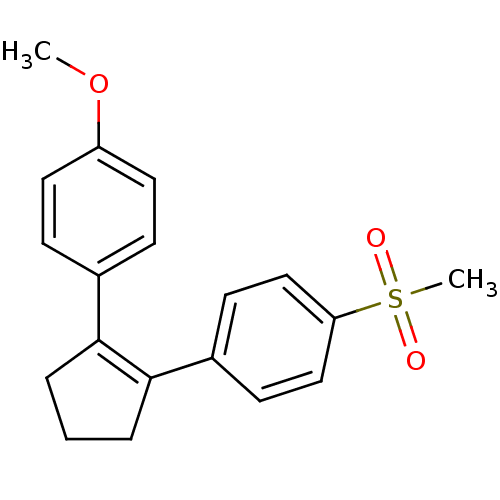
(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029618

(4-(2-(4-methoxyphenyl)cyclopent-1-enyl)benzenesulf...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H19NO3S/c1-22-15-9-5-13(6-10-15)17-3-2-4-18(17)14-7-11-16(12-8-14)23(19,20)21/h5-12H,2-4H2,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029596
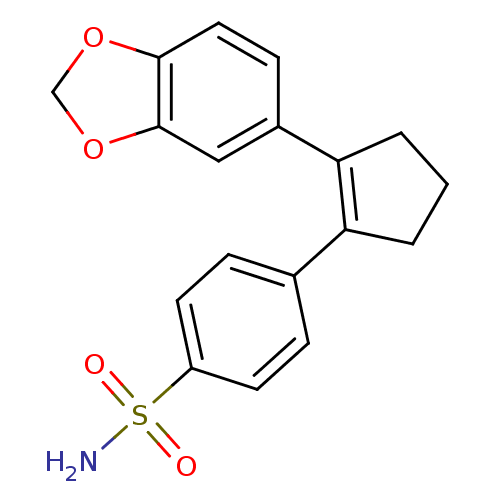
(4-(2-Benzo[1,3]dioxol-5-yl-cyclopent-1-enyl)-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C18H17NO4S/c19-24(20,21)14-7-4-12(5-8-14)15-2-1-3-16(15)13-6-9-17-18(10-13)23-11-22-17/h4-10H,1-3,11H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029595
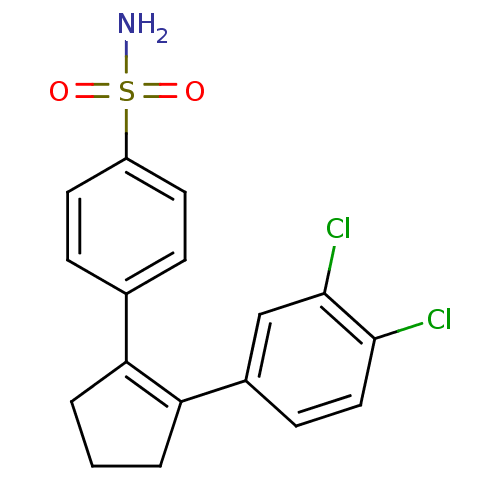
(4-[2-(3,4-Dichloro-phenyl)-cyclopent-1-enyl]-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C17H15Cl2NO2S/c18-16-9-6-12(10-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029624
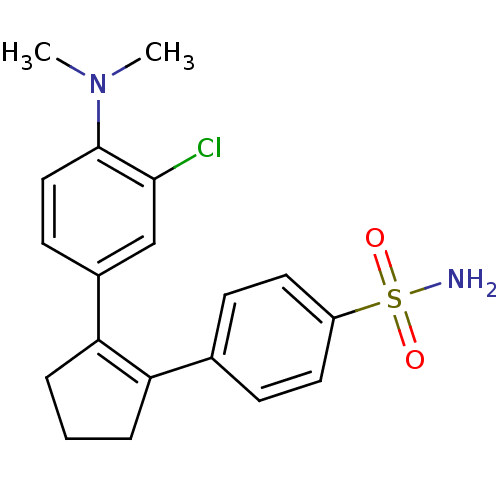
(4-[2-(3-Chloro-4-dimethylamino-phenyl)-cyclopent-1...)Show SMILES CN(C)c1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:11| Show InChI InChI=1S/C19H21ClN2O2S/c1-22(2)19-11-8-14(12-18(19)20)17-5-3-4-16(17)13-6-9-15(10-7-13)25(21,23)24/h6-12H,3-5H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029615

(4-[2-(4-Chloro-phenyl)-cyclopent-1-enyl]-benzenesu...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C17H16ClNO2S/c18-14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)22(19,20)21/h4-11H,1-3H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029623
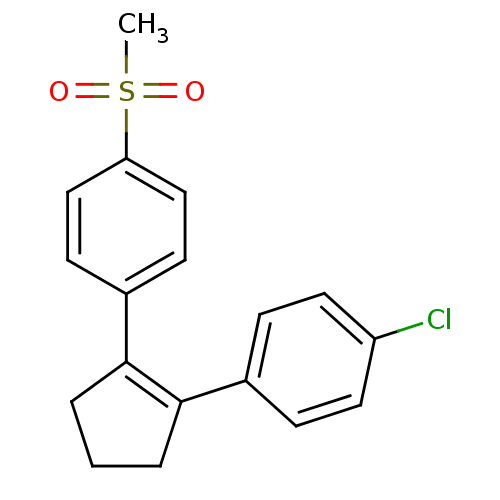
(1-chloro-4-[2-(4-methylsulfonylphenyl)-1-cyclopent...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C18H17ClO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029625

(1-methyl-4-[2-(4-methylsulfonylphenyl)-1-cyclopent...)Show SMILES Cc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:8| Show InChI InChI=1S/C19H20O2S/c1-14-6-8-15(9-7-14)18-4-3-5-19(18)16-10-12-17(13-11-16)22(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029601
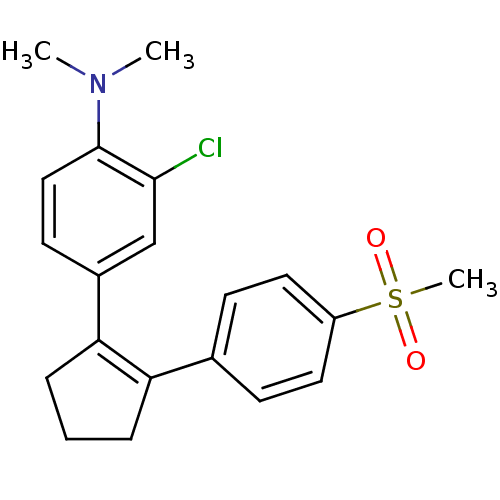
(CHEMBL109623 | N-(2-chloro-4-{2-[4-(methylsulfonyl...)Show SMILES CN(C)c1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C20H22ClNO2S/c1-22(2)20-12-9-15(13-19(20)21)18-6-4-5-17(18)14-7-10-16(11-8-14)25(3,23)24/h7-13H,4-6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029626

(4-[2-(4-Fluoro-phenyl)-cyclopent-1-enyl]-benzenesu...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C17H16FNO2S/c18-14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)22(19,20)21/h4-11H,1-3H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029622

(4-[2-(3-Chloro-4-methoxy-phenyl)-cyclopent-1-enyl]...)Show SMILES COc1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C18H18ClNO3S/c1-23-18-10-7-13(11-17(18)19)16-4-2-3-15(16)12-5-8-14(9-6-12)24(20,21)22/h5-11H,2-4H2,1H3,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029607
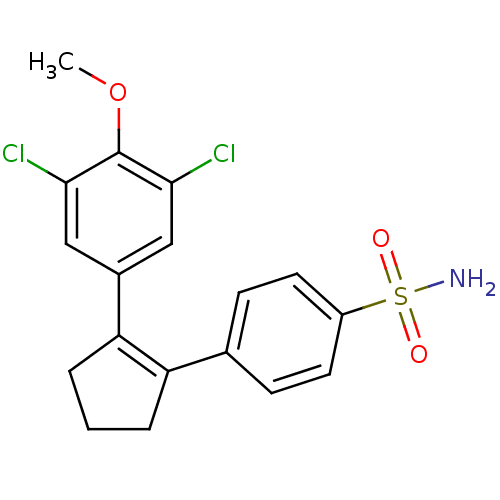
(4-[2-(3,5-Dichloro-4-methoxy-phenyl)-cyclopent-1-e...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:11| Show InChI InChI=1S/C18H17Cl2NO3S/c1-24-18-16(19)9-12(10-17(18)20)15-4-2-3-14(15)11-5-7-13(8-6-11)25(21,22)23/h5-10H,2-4H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029600
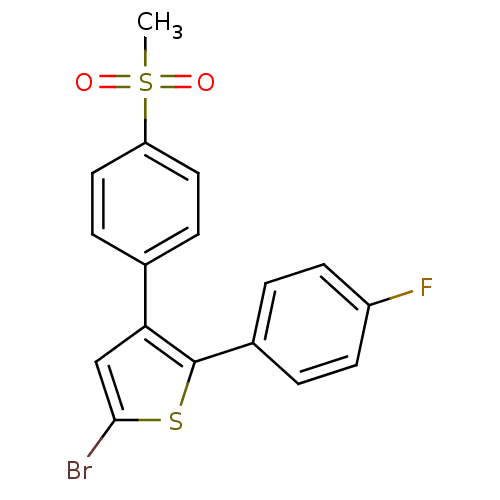
(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029609

(1,2-Dichloro-4-[2-(4-methanesulfonyl-phenyl)-cyclo...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C18H16Cl2O2S/c1-23(21,22)14-8-5-12(6-9-14)15-3-2-4-16(15)13-7-10-17(19)18(20)11-13/h5-11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029611

(4-[2-(3-Chloro-4-fluoro-phenyl)-cyclopent-1-enyl]-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C17H15ClFNO2S/c18-16-10-12(6-9-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029619

(4-[2-(3-Fluoro-4-methoxy-phenyl)-cyclopent-1-enyl]...)Show SMILES COc1ccc(cc1F)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C18H18FNO3S/c1-23-18-10-7-13(11-17(18)19)16-4-2-3-15(16)12-5-8-14(9-6-12)24(20,21)22/h5-11H,2-4H2,1H3,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029599

(1,3-Dichloro-5-[2-(4-methanesulfonyl-phenyl)-cyclo...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C19H18Cl2O3S/c1-24-19-17(20)10-13(11-18(19)21)16-5-3-4-15(16)12-6-8-14(9-7-12)25(2,22)23/h6-11H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029617
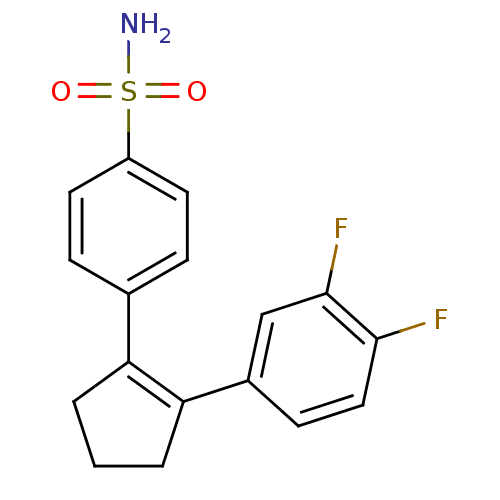
(4-[2-(3,4-Difluoro-phenyl)-cyclopent-1-enyl]-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C17H15F2NO2S/c18-16-9-6-12(10-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029603
(CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...)Show InChI InChI=1S/C16H13F2NO3S2/c1-24(21,22)19-13-6-9-2-4-14(20)11(9)8-16(13)23-15-5-3-10(17)7-12(15)18/h3,5-8,19H,2,4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029606

(5-[2-(4-Methanesulfonyl-phenyl)-cyclopent-1-enyl]-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C19H18O4S/c1-24(20,21)15-8-5-13(6-9-15)16-3-2-4-17(16)14-7-10-18-19(11-14)23-12-22-18/h5-11H,2-4,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029614
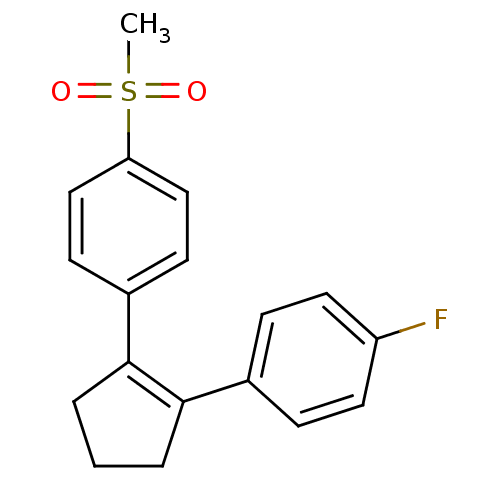
((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C18H17FO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029598
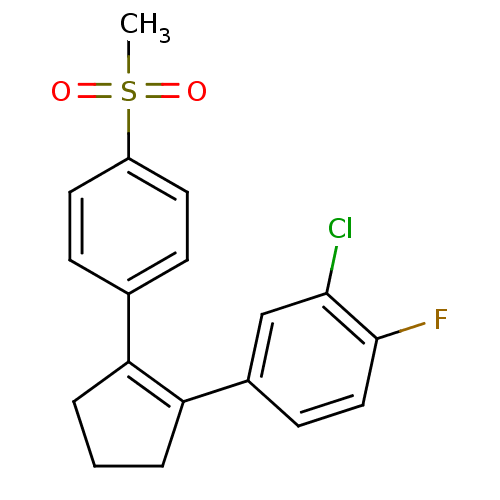
(2-Chloro-1-fluoro-4-[2-(4-methanesulfonyl-phenyl)-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C18H16ClFO2S/c1-23(21,22)14-8-5-12(6-9-14)15-3-2-4-16(15)13-7-10-18(20)17(19)11-13/h5-11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029616
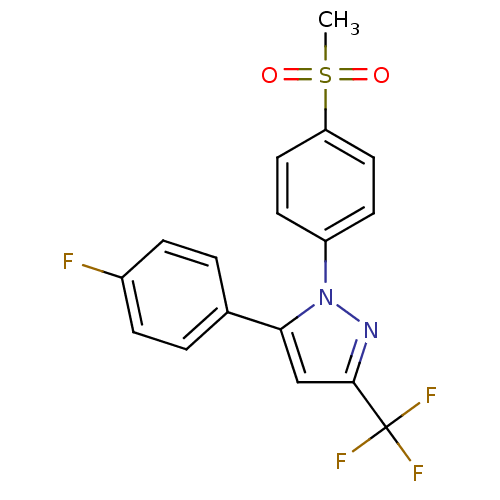
(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029604

(1,2-Difluoro-4-[2-(4-methanesulfonyl-phenyl)-cyclo...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C18H16F2O2S/c1-23(21,22)14-8-5-12(6-9-14)15-3-2-4-16(15)13-7-10-17(19)18(20)11-13/h5-11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029602

(2-Fluoro-4-[2-(4-methanesulfonyl-phenyl)-cyclopent...)Show SMILES COc1ccc(cc1F)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C19H19FO3S/c1-23-19-11-8-14(12-18(19)20)17-5-3-4-16(17)13-6-9-15(10-7-13)24(2,21)22/h6-12H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029620
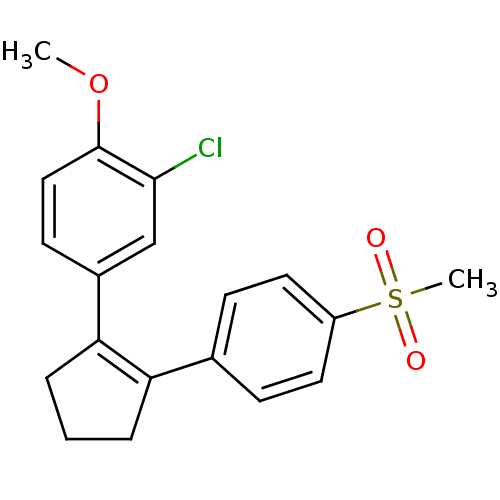
(2-Chloro-4-[2-(4-methanesulfonyl-phenyl)-cyclopent...)Show SMILES COc1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C19H19ClO3S/c1-23-19-11-8-14(12-18(19)20)17-5-3-4-16(17)13-6-9-15(10-7-13)24(2,21)22/h6-12H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029612

(4-[2-(4-Trifluoromethyl-phenyl)-cyclopent-1-enyl]-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C18H16F3NO2S/c19-18(20,21)14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)25(22,23)24/h4-11H,1-3H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029605

(4-[2-(3-Fluoro-4-trifluoromethyl-phenyl)-cyclopent...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(c(F)c1)C(F)(F)F |t:11| Show InChI InChI=1S/C18H15F4NO2S/c19-17-10-12(6-9-16(17)18(20,21)22)15-3-1-2-14(15)11-4-7-13(8-5-11)26(23,24)25/h4-10H,1-3H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029618

(4-(2-(4-methoxyphenyl)cyclopent-1-enyl)benzenesulf...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H19NO3S/c1-22-15-9-5-13(6-10-15)17-3-2-4-18(17)14-7-11-16(12-8-14)23(19,20)21/h5-12H,2-4H2,1H3,(H2,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029608
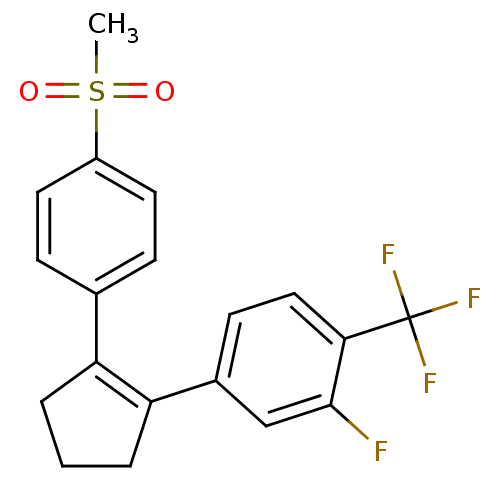
(2-Fluoro-4-[2-(4-methanesulfonyl-phenyl)-cyclopent...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(c(F)c1)C(F)(F)F |t:11| Show InChI InChI=1S/C19H16F4O2S/c1-26(24,25)14-8-5-12(6-9-14)15-3-2-4-16(15)13-7-10-17(18(20)11-13)19(21,22)23/h5-11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029600

(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029610

(1-(methylsulfonyl)-4-{2-[4-(trifluoromethyl)phenyl...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C19H17F3O2S/c1-25(23,24)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(10-6-13)19(20,21)22/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029615

(4-[2-(4-Chloro-phenyl)-cyclopent-1-enyl]-benzenesu...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C17H16ClNO2S/c18-14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)22(19,20)21/h4-11H,1-3H2,(H2,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029624

(4-[2-(3-Chloro-4-dimethylamino-phenyl)-cyclopent-1...)Show SMILES CN(C)c1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:11| Show InChI InChI=1S/C19H21ClN2O2S/c1-22(2)19-11-8-14(12-18(19)20)17-5-3-4-16(17)13-6-9-15(10-7-13)25(21,23)24/h6-12H,3-5H2,1-2H3,(H2,21,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029596

(4-(2-Benzo[1,3]dioxol-5-yl-cyclopent-1-enyl)-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C18H17NO4S/c19-24(20,21)14-7-4-12(5-8-14)15-2-1-3-16(15)13-6-9-17-18(10-13)23-11-22-17/h4-10H,1-3,11H2,(H2,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029594
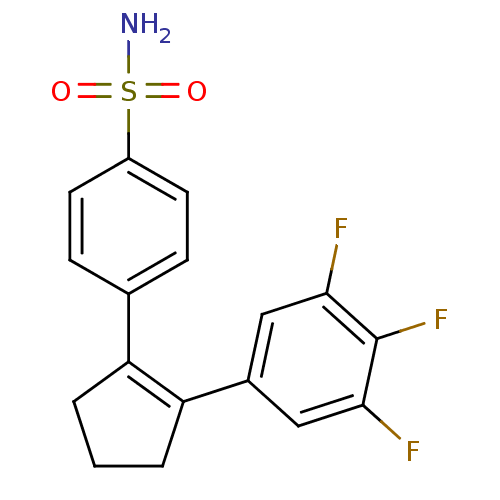
(4-[2-(3,4,5-Trifluoro-phenyl)-cyclopent-1-enyl]-be...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1cc(F)c(F)c(F)c1 |t:11| Show InChI InChI=1S/C17H14F3NO2S/c18-15-8-11(9-16(19)17(15)20)14-3-1-2-13(14)10-4-6-12(7-5-10)24(21,22)23/h4-9H,1-3H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029595

(4-[2-(3,4-Dichloro-phenyl)-cyclopent-1-enyl]-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C17H15Cl2NO2S/c18-16-9-6-12(10-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029612

(4-[2-(4-Trifluoromethyl-phenyl)-cyclopent-1-enyl]-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C18H16F3NO2S/c19-18(20,21)14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)25(22,23)24/h4-11H,1-3H2,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029626

(4-[2-(4-Fluoro-phenyl)-cyclopent-1-enyl]-benzenesu...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C17H16FNO2S/c18-14-8-4-12(5-9-14)16-2-1-3-17(16)13-6-10-15(11-7-13)22(19,20)21/h4-11H,1-3H2,(H2,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029611

(4-[2-(3-Chloro-4-fluoro-phenyl)-cyclopent-1-enyl]-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C17H15ClFNO2S/c18-16-10-12(6-9-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029617

(4-[2-(3,4-Difluoro-phenyl)-cyclopent-1-enyl]-benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C17H15F2NO2S/c18-16-9-6-12(10-17(16)19)15-3-1-2-14(15)11-4-7-13(8-5-11)23(20,21)22/h4-10H,1-3H2,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029619

(4-[2-(3-Fluoro-4-methoxy-phenyl)-cyclopent-1-enyl]...)Show SMILES COc1ccc(cc1F)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C18H18FNO3S/c1-23-18-10-7-13(11-17(18)19)16-4-2-3-15(16)12-5-8-14(9-6-12)24(20,21)22/h5-11H,2-4H2,1H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029613

(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029603

(CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...)Show InChI InChI=1S/C16H13F2NO3S2/c1-24(21,22)19-13-6-9-2-4-14(20)11(9)8-16(13)23-15-5-3-10(17)7-12(15)18/h3,5-8,19H,2,4H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029605

(4-[2-(3-Fluoro-4-trifluoromethyl-phenyl)-cyclopent...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(c(F)c1)C(F)(F)F |t:11| Show InChI InChI=1S/C18H15F4NO2S/c19-17-10-12(6-9-16(17)18(20,21)22)15-3-1-2-14(15)11-4-7-13(8-5-11)26(23,24)25/h4-10H,1-3H2,(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029622

(4-[2-(3-Chloro-4-methoxy-phenyl)-cyclopent-1-enyl]...)Show SMILES COc1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C18H18ClNO3S/c1-23-18-10-7-13(11-17(18)19)16-4-2-3-15(16)12-5-8-14(9-6-12)24(20,21)22/h5-11H,2-4H2,1H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029601

(CHEMBL109623 | N-(2-chloro-4-{2-[4-(methylsulfonyl...)Show SMILES CN(C)c1ccc(cc1Cl)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C20H22ClNO2S/c1-22(2)20-12-9-15(13-19(20)21)18-6-4-5-17(18)14-7-10-16(11-8-14)25(3,23)24/h7-13H,4-6H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 1. |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data