

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061511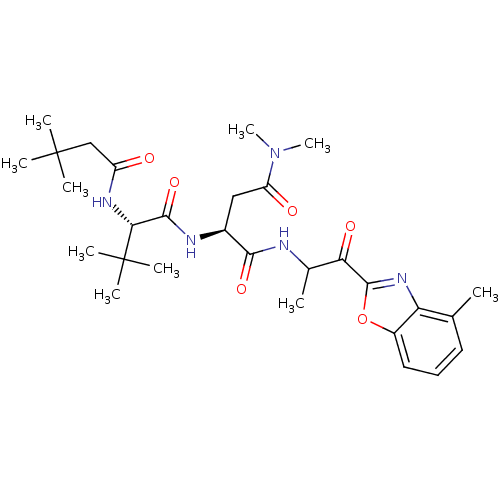 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061512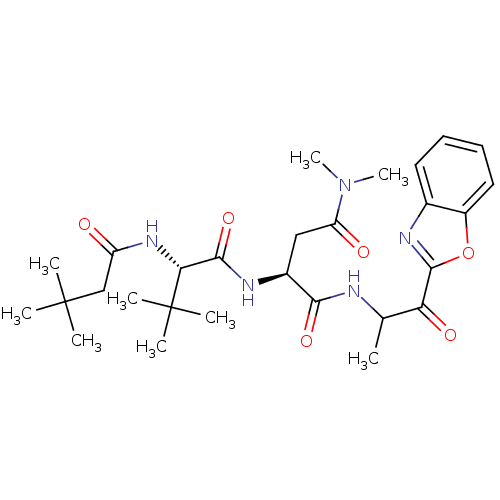 ((S)-N*1*-(2-Benzooxazol-2-yl-1-methyl-2-oxo-ethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061532 ((S)-N*1*-{2-[(Benzo[1,3]dioxol-5-ylmethyl)-carbamo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061537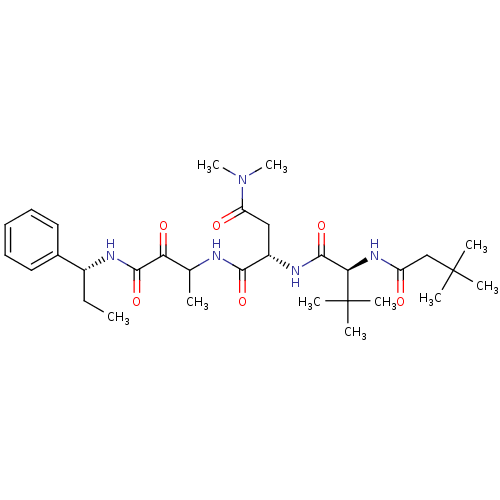 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061518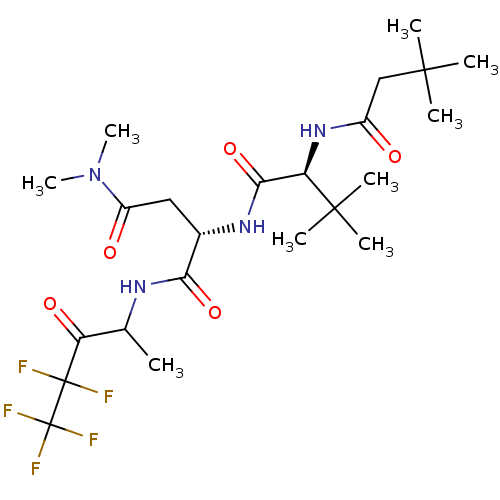 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061515 ((S)-N*1*-[2-(2-Benzyloxy-ethylcarbamoyl)-1-methyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061509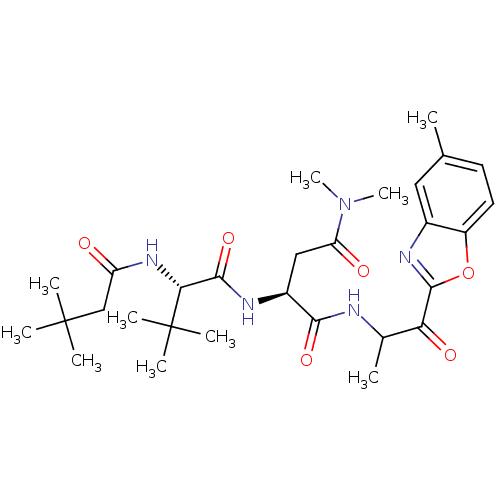 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061503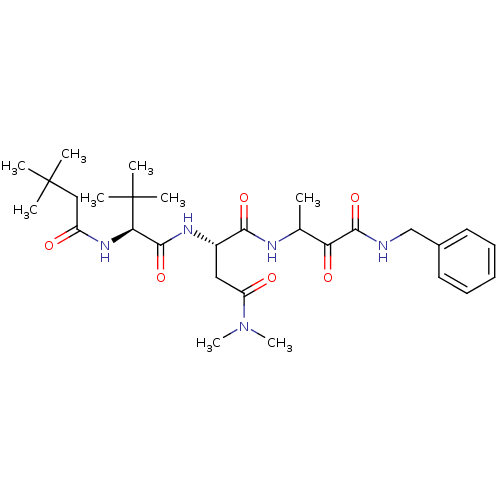 ((S)-N*1*-(2-Benzylcarbamoyl-1-methyl-2-oxo-ethyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061528 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061506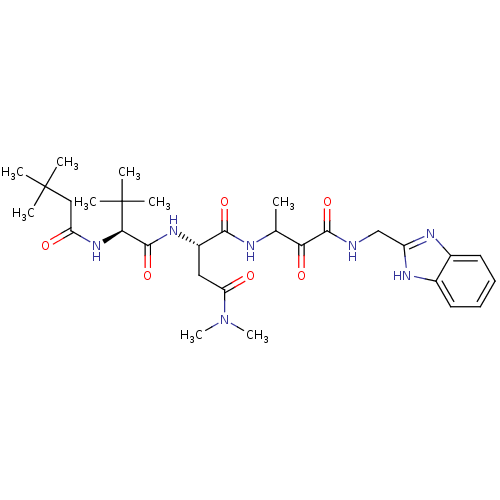 ((S)-N*1*-{2-[(1H-Benzoimidazol-2-ylmethyl)-carbamo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061544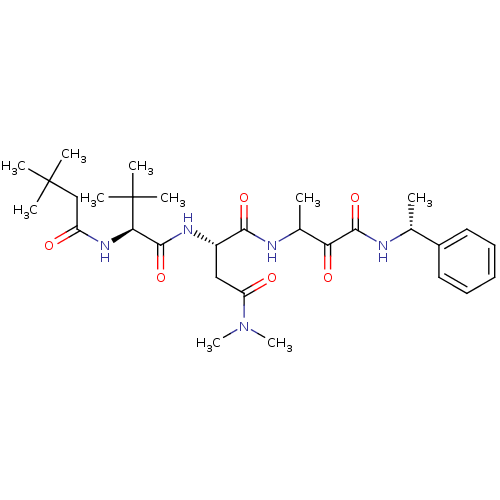 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061497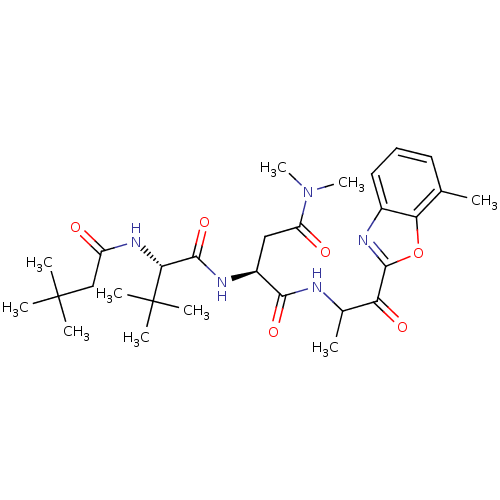 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061530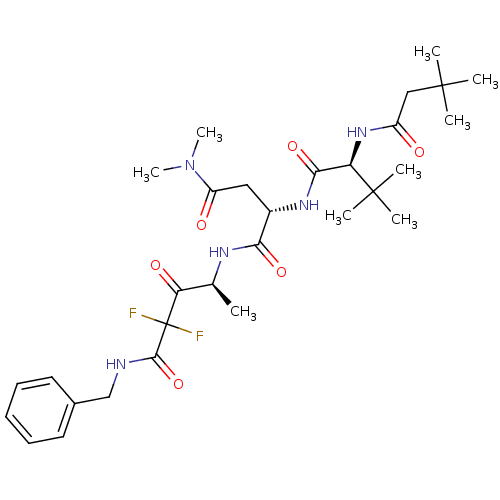 ((S)-N*1*-((S)-3-Benzylcarbamoyl-3,3-difluoro-1-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061528 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061512 ((S)-N*1*-(2-Benzooxazol-2-yl-1-methyl-2-oxo-ethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061509 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061516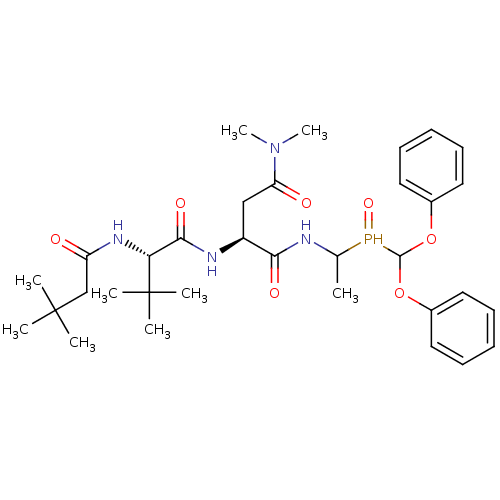 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061511 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061551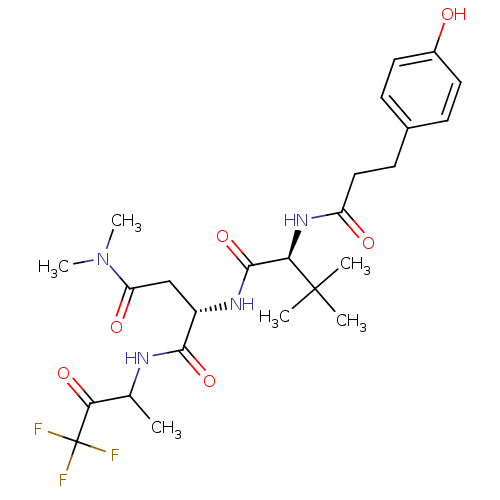 ((S)-2-{(S)-2-[3-(4-Hydroxy-phenyl)-propionylamino]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061498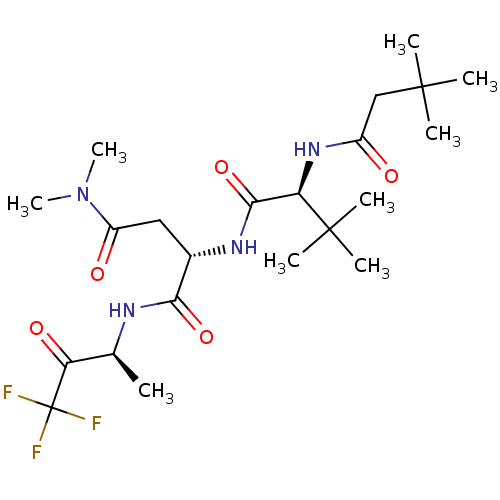 ((S)-2-((S)-2-(3,3-dimethylbutanamido)-3,3-dimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061553 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061505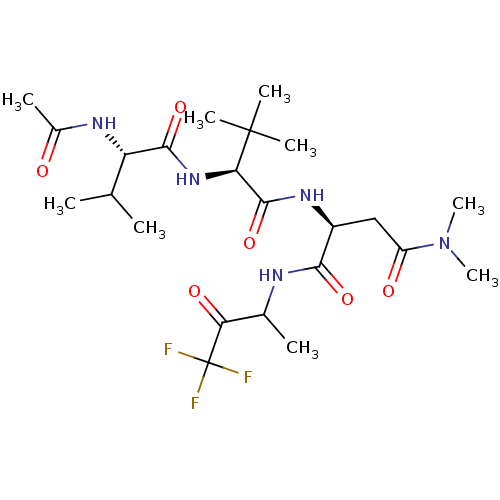 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061510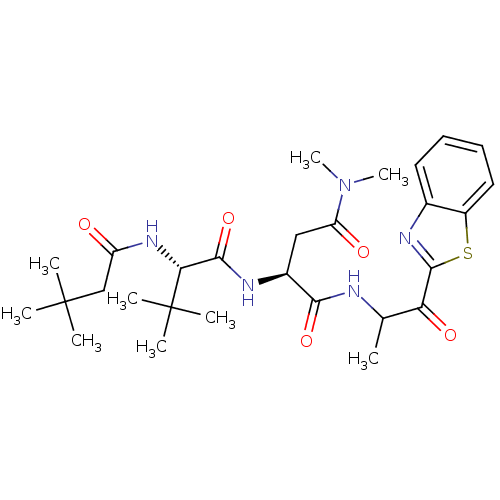 ((S)-N*1*-(2-Benzothiazol-2-yl-1-methyl-2-oxo-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061540 ((S)-2-[(S)-3,3-Dimethyl-2-(3-methyl-butyrylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061534 (4-{(S)-1-[(S)-2-Dimethylcarbamoyl-1-(3,3,3-trifluo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061526 ((S)-2-{(S)-2-[(S)-2-(2-Acetylamino-acetylamino)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061538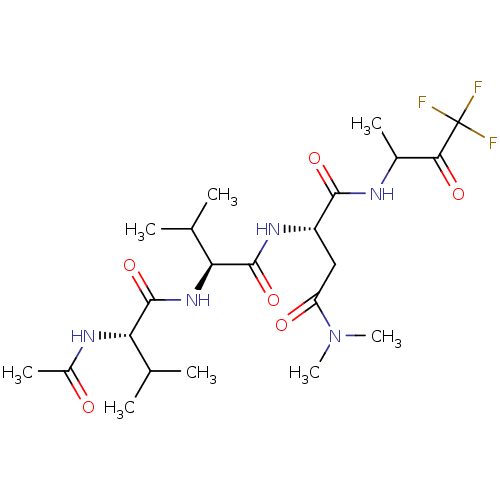 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061523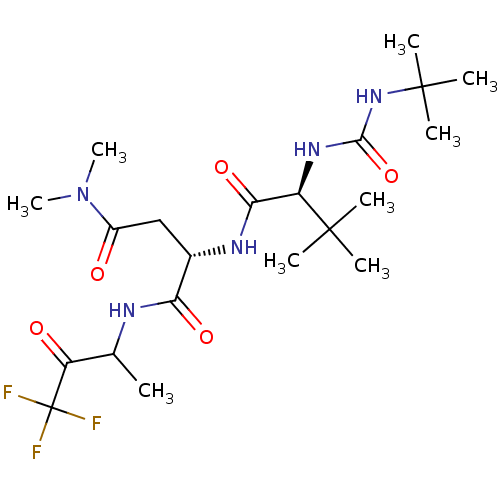 ((S)-2-[(S)-2-(3-tert-Butyl-ureido)-3,3-dimethyl-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061521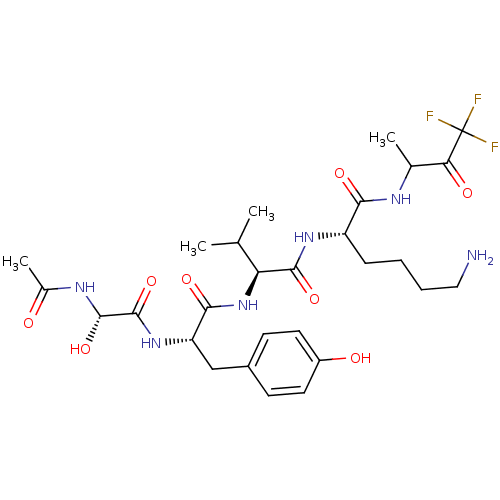 ((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-2-hydroxy-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061546 ((S)-2-((S)-2-Acetylamino-3,3-dimethyl-butyrylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061497 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061522 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061520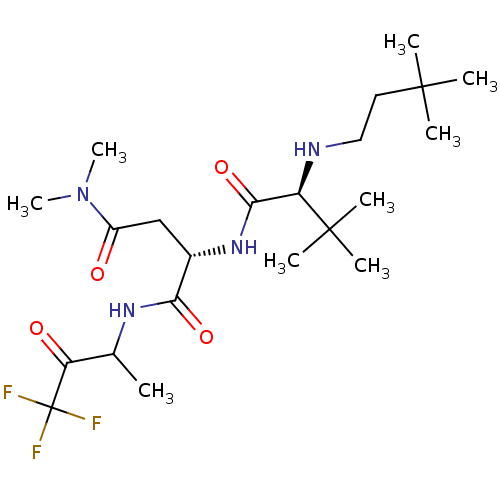 ((S)-2-[(S)-2-(3,3-Dimethyl-butylamino)-3,3-dimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061536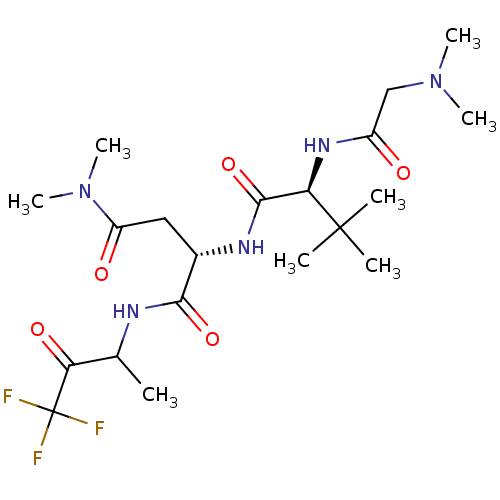 ((S)-2-[(S)-2-(2-Dimethylamino-acetylamino)-3,3-dim...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061531 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061539 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061514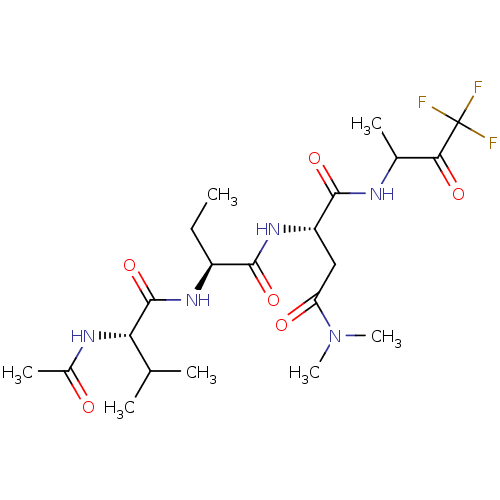 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061503 ((S)-N*1*-(2-Benzylcarbamoyl-1-methyl-2-oxo-ethyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061542 ((S)-2-Acetylamino-3-methyl-N-{(S)-2-methyl-1-[(S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061547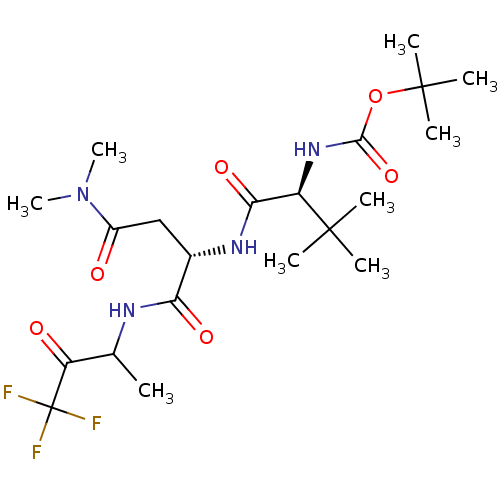 (CHEMBL133346 | {(S)-1-[(S)-2-Dimethylcarbamoyl-1-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061541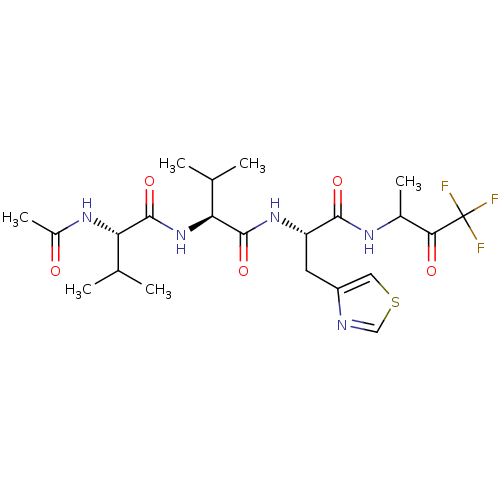 ((S)-2-Acetylamino-3-methyl-N-{(S)-2-methyl-1-[(S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061502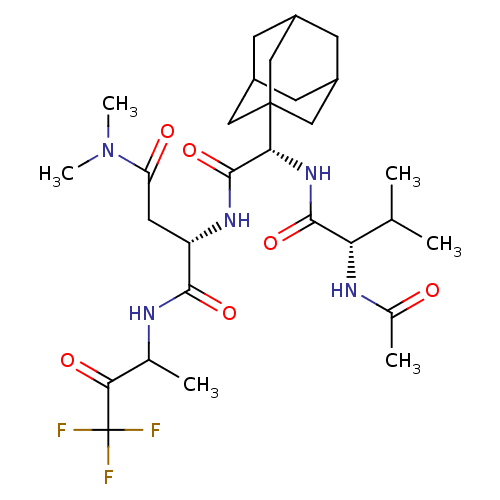 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061517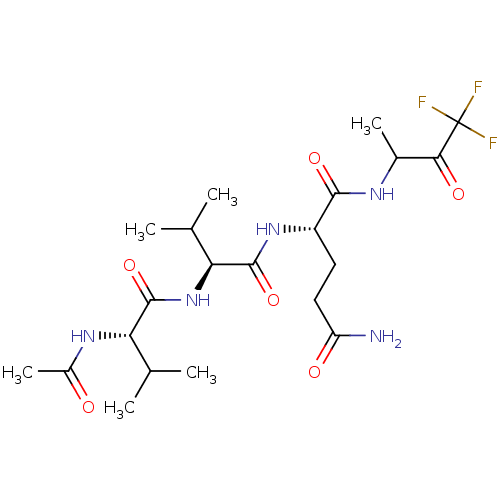 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061508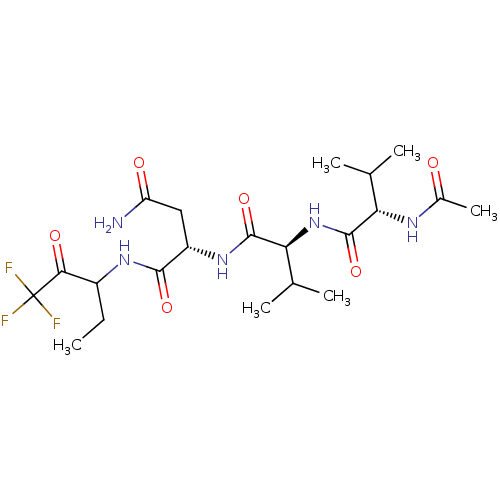 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061510 ((S)-N*1*-(2-Benzothiazol-2-yl-1-methyl-2-oxo-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061500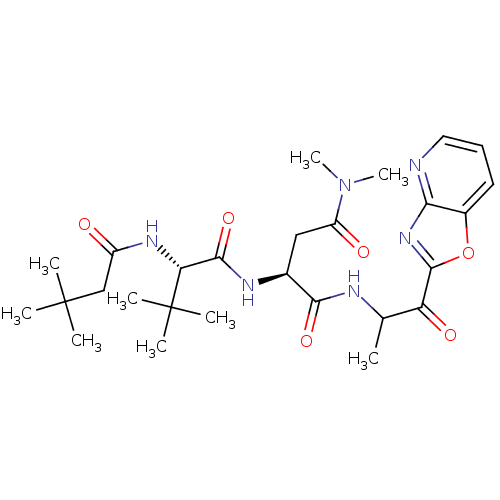 ((S)-2-((S)-2-(3,3-dimethylbutanamido)-3,3-dimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061529 ((S)-2-[(S)-2-((S)-2-Acetylamino-3-methyl-butyrylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061501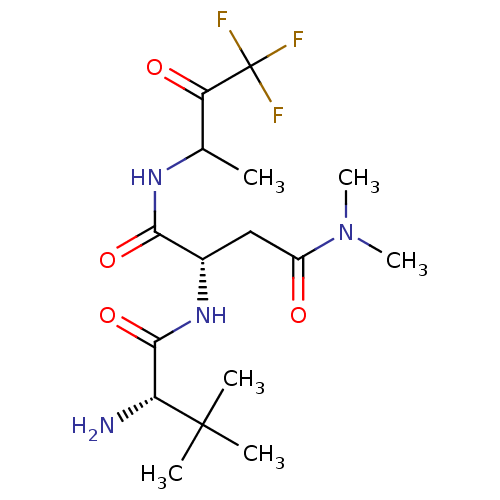 ((S)-2-((S)-2-Amino-3,3-dimethyl-butyrylamino)-N*4*...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50061518 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease bovine pancreatic Alpha-Chymotrypsinogen (BPC) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061525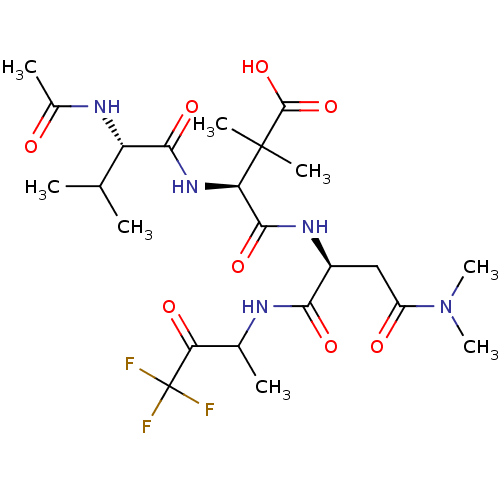 ((S)-3-((S)-2-Acetylamino-3-methyl-butyrylamino)-N-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |