

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062291 (CHEMBL54116 | Phosphoric acid mono-[2-(6-methylami...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118229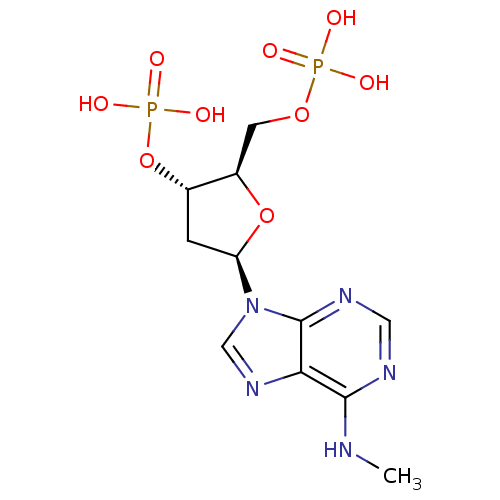 (CHEMBL129841 | MRS 2179) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062276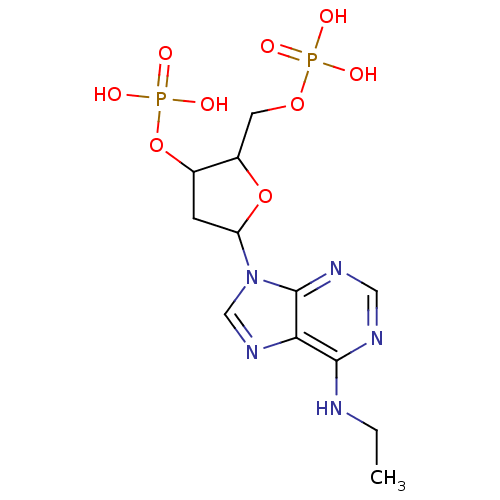 (CHEMBL55247 | Phosphoric acid mono-[5-(6-ethylamin...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286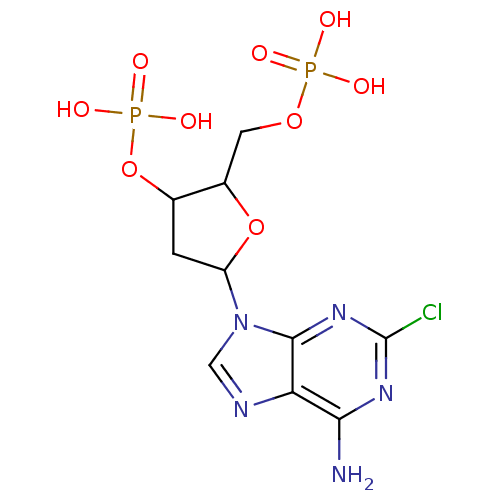 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062283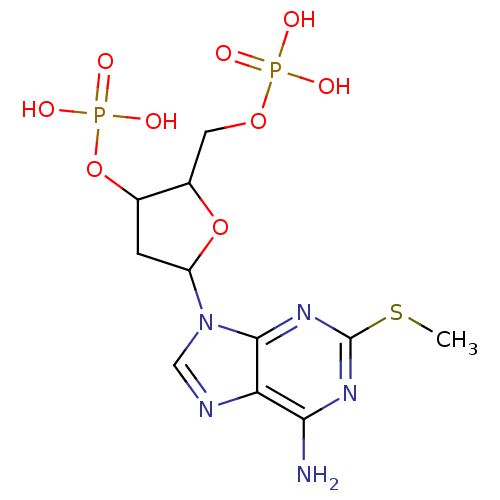 (CHEMBL43531 | Phosphoric acid mono-[5-(6-amino-2-m...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062279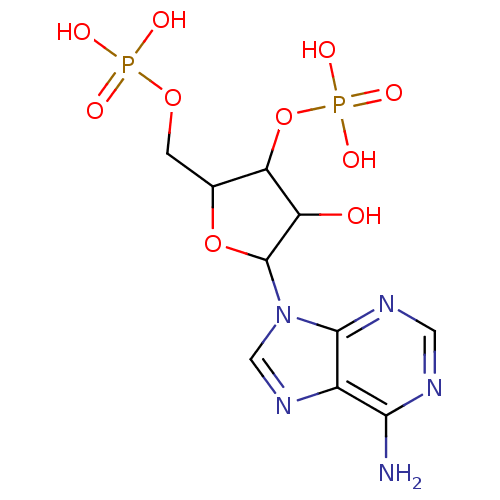 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062287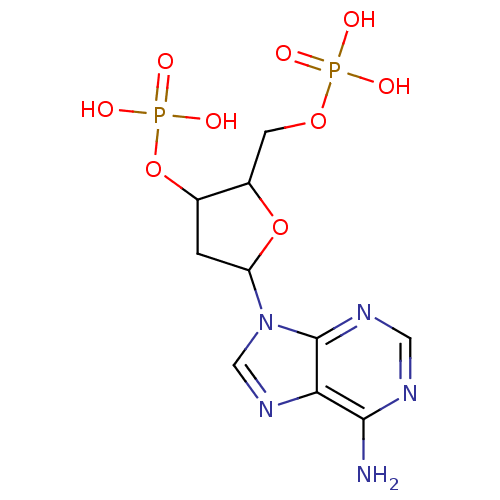 (CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062282 (CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062278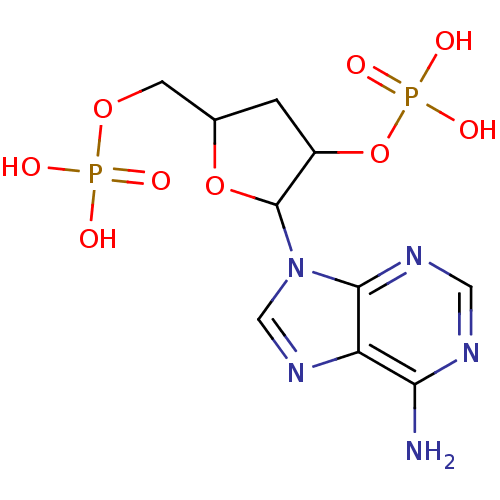 (CHEMBL55804 | Phosphoric acid mono-[2-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062285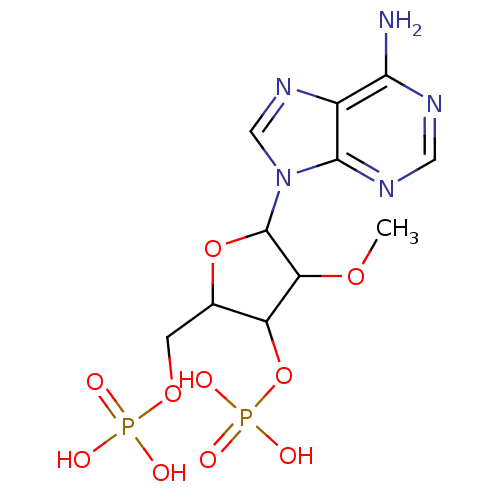 (CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062280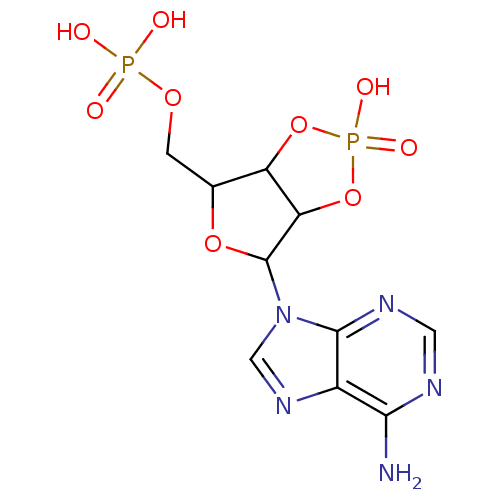 (CHEMBL55718 | Phosphoric acid mono-[6-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062289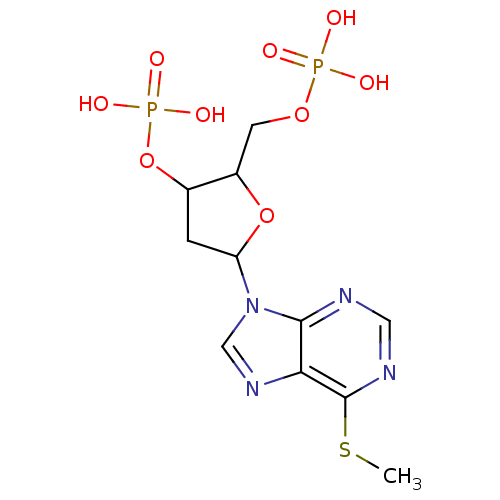 (CHEMBL294601 | Phosphoric acid mono-[5-(6-methylsu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062288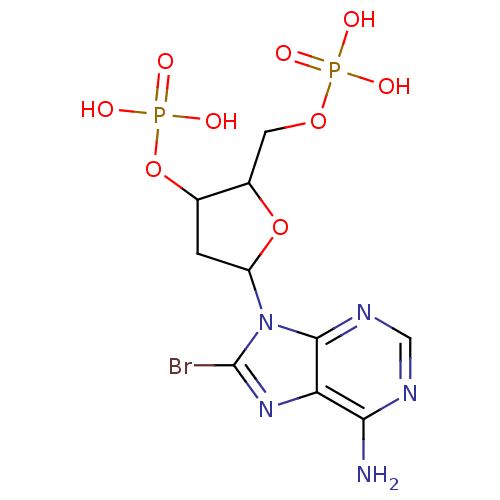 (CHEMBL431941 | Phosphoric acid mono-[5-(6-amino-8-...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062281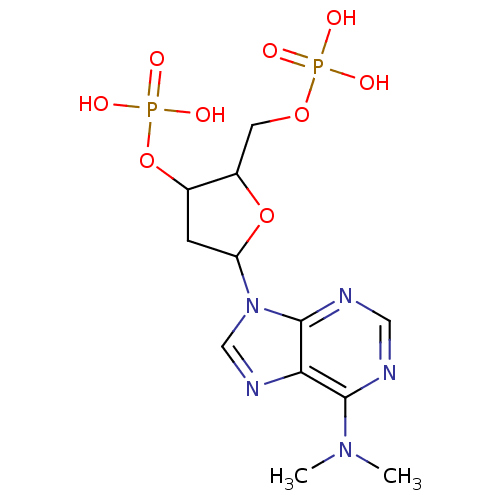 (CHEMBL59090 | Phosphoric acid mono-[5-(6-dimethyla...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062290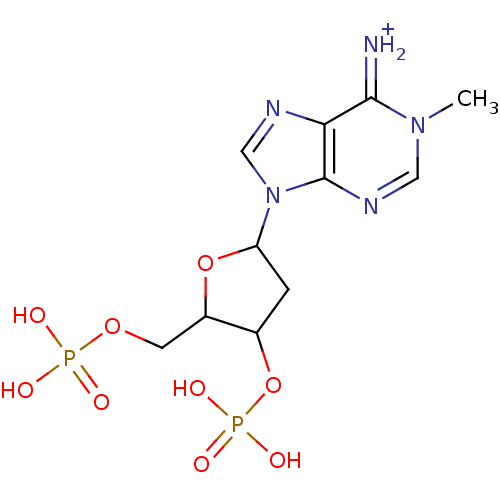 (6-Amino-1-methyl-9-(4-phosphonooxy-5-phosphonooxym...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062275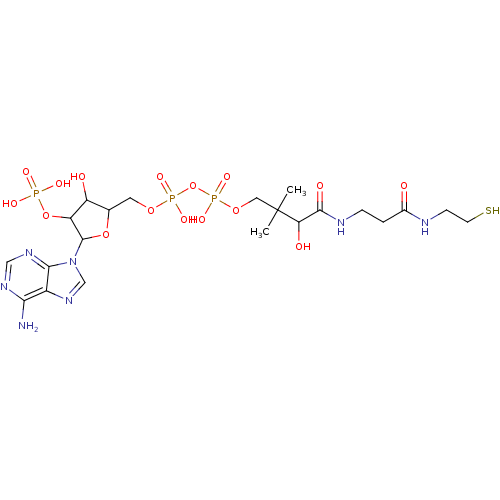 (CHEMBL292013 | {[5-(6-Amino-purin-9-yl)-3-hydroxy-...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062284 (CHEMBL56787 | Thiophosphoric acid 5-(6-amino-purin...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50369391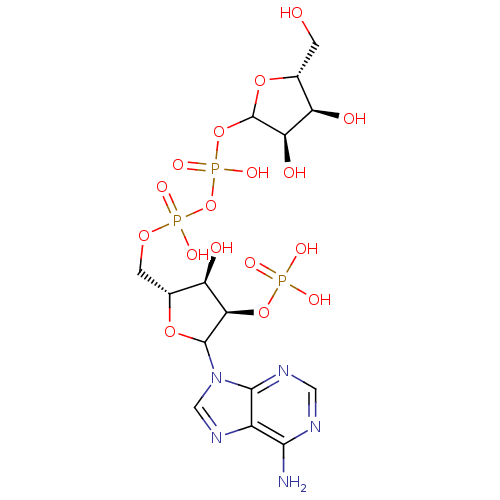 (CHEMBL608872) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062278 (CHEMBL55804 | Phosphoric acid mono-[2-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062285 (CHEMBL292442 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062282 (CHEMBL58764 | Phosphoric acid mono-[2-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062287 (CHEMBL298487 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.26E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062280 (CHEMBL55718 | Phosphoric acid mono-[6-(6-amino-pur...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062275 (CHEMBL292013 | {[5-(6-Amino-purin-9-yl)-3-hydroxy-...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50369391 (CHEMBL608872) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 651 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062283 (CHEMBL43531 | Phosphoric acid mono-[5-(6-amino-2-m...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||