 Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50006185
Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50006185 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045797
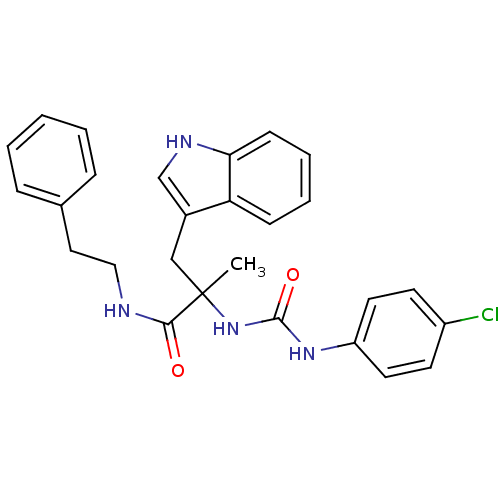
(2-[3-(4-Chloro-phenyl)-ureido]-3-(1H-indol-3-yl)-2...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(Cl)cc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C27H27ClN4O2/c1-27(17-20-18-30-24-10-6-5-9-23(20)24,25(33)29-16-15-19-7-3-2-4-8-19)32-26(34)31-22-13-11-21(28)12-14-22/h2-14,18,30H,15-17H2,1H3,(H,29,33)(H2,31,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045805
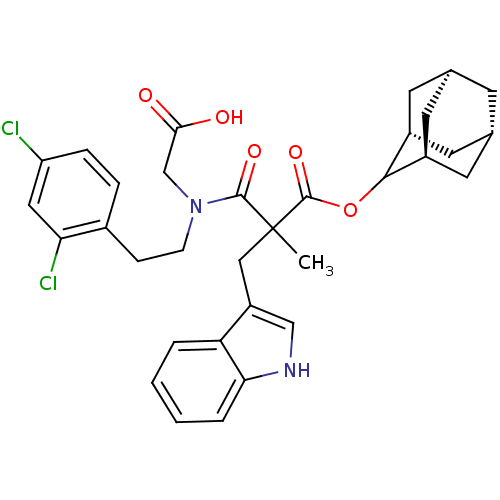
(CHEMBL318852 | N-Carboxymethyl-N-[2-(2,4-dichloro-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(C(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:20.27,18.19,22.23,wD:16.28,TLB:14:15:21:18.23.19,THB:17:16:21:18.23.19,17:18:21:15.16.24,(15.87,-6.38,;14.54,-7.15,;13.21,-7.93,;13.23,-9.47,;14.14,-10.71,;13.24,-11.97,;11.77,-11.5,;10.41,-12.27,;9.08,-11.5,;9.08,-9.96,;10.41,-9.18,;11.76,-9.95,;13.04,-6.75,;11.95,-7.82,;12.65,-5.26,;11.16,-4.86,;9.83,-4.06,;9.27,-2.64,;7.64,-2.8,;6.52,-3.85,;7.08,-5.26,;8.35,-5.9,;9.41,-4.86,;8.85,-3.57,;8.69,-5.16,;15.87,-7.92,;15.87,-9.46,;17.41,-7.92,;18.18,-6.58,;19.72,-6.58,;20.49,-5.23,;19.72,-3.92,;20.47,-2.59,;22.03,-2.57,;22.78,-1.24,;22.8,-3.92,;22.03,-5.25,;22.8,-6.58,;18.18,-9.25,;19.72,-9.25,;20.49,-10.58,;20.49,-7.91,)| Show InChI InChI=1S/C33H36Cl2N2O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,32(41)42-30-22-11-19-10-20(13-22)14-23(30)12-19)31(40)37(18-29(38)39)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,38,39)/t19-,20+,22-,23+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50045805

(CHEMBL318852 | N-Carboxymethyl-N-[2-(2,4-dichloro-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(C(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:20.27,18.19,22.23,wD:16.28,TLB:14:15:21:18.23.19,THB:17:16:21:18.23.19,17:18:21:15.16.24,(15.87,-6.38,;14.54,-7.15,;13.21,-7.93,;13.23,-9.47,;14.14,-10.71,;13.24,-11.97,;11.77,-11.5,;10.41,-12.27,;9.08,-11.5,;9.08,-9.96,;10.41,-9.18,;11.76,-9.95,;13.04,-6.75,;11.95,-7.82,;12.65,-5.26,;11.16,-4.86,;9.83,-4.06,;9.27,-2.64,;7.64,-2.8,;6.52,-3.85,;7.08,-5.26,;8.35,-5.9,;9.41,-4.86,;8.85,-3.57,;8.69,-5.16,;15.87,-7.92,;15.87,-9.46,;17.41,-7.92,;18.18,-6.58,;19.72,-6.58,;20.49,-5.23,;19.72,-3.92,;20.47,-2.59,;22.03,-2.57,;22.78,-1.24,;22.8,-3.92,;22.03,-5.25,;22.8,-6.58,;18.18,-9.25,;19.72,-9.25,;20.49,-10.58,;20.49,-7.91,)| Show InChI InChI=1S/C33H36Cl2N2O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,32(41)42-30-22-11-19-10-20(13-22)14-23(30)12-19)31(40)37(18-29(38)39)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,38,39)/t19-,20+,22-,23+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in mouse brain by displacement of [3H]-pBC264 radioligand (icv) |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type A receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045800
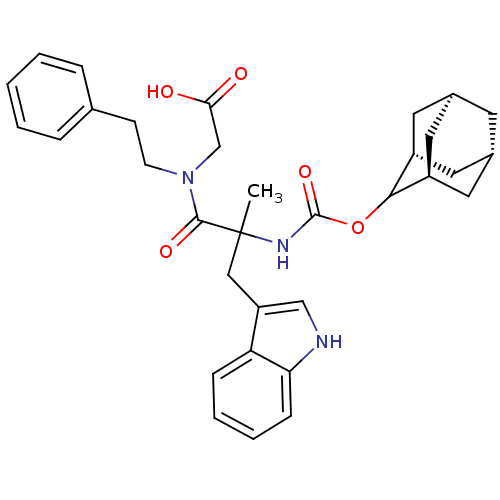
(CHEMBL317999 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(6.49,-7.36,;5.16,-8.14,;3.83,-8.91,;3.83,-10.46,;4.74,-11.7,;3.85,-12.96,;2.38,-12.49,;1.03,-13.26,;-.3,-12.49,;-.3,-10.94,;1.03,-10.17,;2.36,-10.94,;3.81,-7.37,;2.48,-6.6,;1.38,-7.68,;2.08,-5.11,;.59,-4.71,;-.74,-3.9,;-1.3,-2.5,;-2.93,-2.64,;-4.05,-3.69,;-3.49,-5.11,;-2.22,-5.75,;-1.14,-4.72,;-1.72,-3.41,;-1.88,-5.02,;6.49,-8.9,;6.49,-10.44,;8.03,-8.9,;8.8,-7.57,;10.34,-7.57,;11.11,-6.23,;10.32,-4.91,;11.09,-3.58,;12.63,-3.57,;13.42,-4.91,;12.65,-6.24,;8.8,-10.23,;10.34,-10.23,;11.11,-11.56,;11.11,-8.9,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-26-19-34-28-10-6-5-9-27(26)28,31(39)36(20-29(37)38)12-11-21-7-3-2-4-8-21)35-32(40)41-30-24-14-22-13-23(16-24)17-25(30)15-22/h2-10,19,22-25,30,34H,11-18,20H2,1H3,(H,35,40)(H,37,38)/t22-,23+,24-,25+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards CCK-B in rat cortex by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in rat cortex by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards CCK-B in mouse brain by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045798
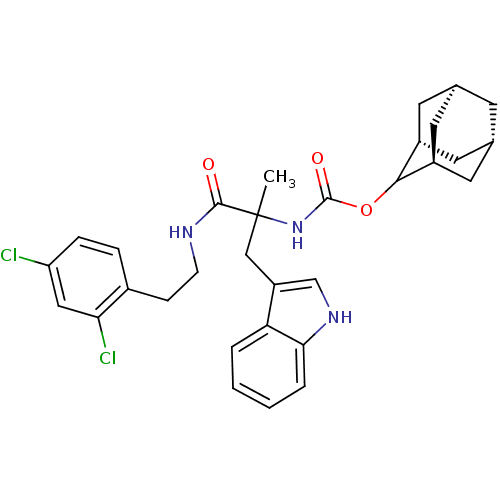
(CHEMBL319120 | [1-[2-(2,4-Dichloro-phenyl)-ethylca...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Cl)cc1Cl |wU:21.28,19.20,23.24,wD:17.29,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(16.99,-12.55,;15.66,-13.33,;14.35,-14.1,;14.36,-15.66,;15.27,-16.89,;14.36,-18.15,;12.89,-17.69,;11.55,-18.46,;10.22,-17.69,;10.22,-16.13,;11.55,-15.36,;12.89,-16.13,;14.17,-12.93,;12.84,-12.16,;11.74,-13.24,;12.44,-10.67,;10.95,-10.27,;9.62,-9.47,;9.06,-8.06,;7.43,-8.21,;6.31,-9.26,;6.87,-10.67,;8.14,-11.32,;9.22,-10.27,;8.64,-8.98,;8.48,-10.58,;17.01,-14.1,;16.99,-15.63,;18.55,-14.09,;19.3,-12.76,;20.84,-12.76,;21.61,-11.42,;20.84,-10.1,;21.61,-8.77,;23.15,-8.76,;23.92,-7.43,;23.92,-10.1,;23.15,-11.43,;23.92,-12.76,)| Show InChI InChI=1S/C31H35Cl2N3O3/c1-31(16-23-17-35-27-5-3-2-4-25(23)27,29(37)34-9-8-20-6-7-24(32)15-26(20)33)36-30(38)39-28-21-11-18-10-19(13-21)14-22(28)12-18/h2-7,15,17-19,21-22,28,35H,8-14,16H2,1H3,(H,34,37)(H,36,38)/t18-,19+,21-,22+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045805

(CHEMBL318852 | N-Carboxymethyl-N-[2-(2,4-dichloro-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(C(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:20.27,18.19,22.23,wD:16.28,TLB:14:15:21:18.23.19,THB:17:16:21:18.23.19,17:18:21:15.16.24,(15.87,-6.38,;14.54,-7.15,;13.21,-7.93,;13.23,-9.47,;14.14,-10.71,;13.24,-11.97,;11.77,-11.5,;10.41,-12.27,;9.08,-11.5,;9.08,-9.96,;10.41,-9.18,;11.76,-9.95,;13.04,-6.75,;11.95,-7.82,;12.65,-5.26,;11.16,-4.86,;9.83,-4.06,;9.27,-2.64,;7.64,-2.8,;6.52,-3.85,;7.08,-5.26,;8.35,-5.9,;9.41,-4.86,;8.85,-3.57,;8.69,-5.16,;15.87,-7.92,;15.87,-9.46,;17.41,-7.92,;18.18,-6.58,;19.72,-6.58,;20.49,-5.23,;19.72,-3.92,;20.47,-2.59,;22.03,-2.57,;22.78,-1.24,;22.8,-3.92,;22.03,-5.25,;22.8,-6.58,;18.18,-9.25,;19.72,-9.25,;20.49,-10.58,;20.49,-7.91,)| Show InChI InChI=1S/C33H36Cl2N2O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,32(41)42-30-22-11-19-10-20(13-22)14-23(30)12-19)31(40)37(18-29(38)39)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,38,39)/t19-,20+,22-,23+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045810

(3-{[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indol...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCC(O)=O)CCc1ccccc1 |wU:23.26,wD:21.23,19.27,17.29,TLB:24:19:25:16.23.22,15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(12.3,-9.53,;10.97,-10.3,;9.66,-11.08,;9.67,-12.62,;10.58,-13.86,;9.67,-15.12,;8.2,-14.65,;6.86,-15.42,;5.53,-14.65,;5.53,-13.11,;6.86,-12.33,;8.2,-13.1,;9.48,-9.9,;8.14,-9.13,;7.05,-10.2,;7.75,-7.64,;6.26,-7.24,;4.93,-6.44,;4.37,-5.02,;2.74,-5.18,;1.62,-6.23,;2.18,-7.64,;3.45,-8.28,;4.51,-7.24,;3.95,-5.95,;3.79,-7.54,;12.32,-11.07,;12.3,-12.61,;13.86,-11.07,;14.63,-12.4,;16.17,-12.4,;16.94,-13.73,;18.48,-13.73,;16.17,-15.06,;14.61,-9.73,;16.15,-9.73,;16.92,-8.38,;16.15,-7.07,;16.92,-5.74,;18.46,-5.72,;19.23,-7.07,;18.46,-8.4,)| Show InChI InChI=1S/C34H41N3O5/c1-34(20-27-21-35-29-10-6-5-9-28(27)29,32(40)37(14-12-30(38)39)13-11-22-7-3-2-4-8-22)36-33(41)42-31-25-16-23-15-24(18-25)19-26(31)17-23/h2-10,21,23-26,31,35H,11-20H2,1H3,(H,36,41)(H,38,39)/t23-,24+,25-,26+,31?,34? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045816
(CHEMBL319880 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:17.19,wD:21.28,19.20,23.26,TLB:25:21:24:16.17.18,15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(25.85,-.15,;24.53,-.92,;23.2,-1.7,;23.22,-3.24,;24.13,-4.48,;23.22,-5.74,;21.75,-5.27,;20.4,-6.04,;19.07,-5.27,;19.07,-3.73,;20.4,-2.95,;21.75,-3.72,;23.03,-.52,;21.68,.25,;20.6,-.82,;21.3,1.74,;19.81,2.14,;18.07,2.14,;16.99,1.1,;15.73,1.74,;15.17,3.15,;16.29,4.2,;17.92,4.36,;18.49,2.94,;17.34,1.84,;17.51,3.43,;25.86,-1.69,;25.85,-3.23,;27.4,-1.69,;28.17,-.35,;29.71,-.35,;30.48,1,;29.7,2.31,;30.46,3.66,;32.01,3.66,;32.77,4.99,;32.79,2.31,;32.02,.98,;32.78,-.35,;28.17,-3.02,;29.71,-3.02,;30.48,-4.35,;30.48,-1.68,)| Show InChI InChI=1S/C33H37Cl2N3O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,37-32(42)43-30-22-11-19-10-20(13-22)14-23(30)12-19)31(41)38(18-29(39)40)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,37,42)(H,39,40)/t19-,20+,22-,23+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045803
(CHEMBL433407 | [1-[2-(4-Chloro-phenyl)-ethylcarbam...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Cl)cc1 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(14.26,-12.21,;12.93,-13,;11.6,-13.77,;11.6,-15.31,;12.51,-16.55,;11.62,-17.81,;10.15,-17.34,;8.8,-18.11,;7.47,-17.34,;7.47,-15.8,;8.8,-15.03,;10.13,-15.8,;11.43,-12.58,;10.09,-11.81,;8.99,-12.9,;9.69,-10.32,;8.21,-9.92,;6.46,-9.94,;5.4,-10.97,;4.11,-10.32,;3.57,-8.92,;4.67,-7.87,;6.31,-7.71,;6.88,-9.13,;5.72,-10.23,;5.9,-8.64,;14.26,-13.75,;14.26,-15.29,;15.8,-13.75,;16.57,-12.42,;18.11,-12.42,;18.88,-11.08,;18.09,-9.76,;18.86,-8.43,;20.4,-8.41,;21.17,-7.08,;21.19,-9.76,;20.42,-11.09,)| Show InChI InChI=1S/C31H36ClN3O3/c1-31(17-24-18-34-27-5-3-2-4-26(24)27,29(36)33-11-10-19-6-8-25(32)9-7-19)35-30(37)38-28-22-13-20-12-21(15-22)16-23(28)14-20/h2-9,18,20-23,28,34H,10-17H2,1H3,(H,33,36)(H,35,37)/t20-,21+,22-,23+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50045800

(CHEMBL317999 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(6.49,-7.36,;5.16,-8.14,;3.83,-8.91,;3.83,-10.46,;4.74,-11.7,;3.85,-12.96,;2.38,-12.49,;1.03,-13.26,;-.3,-12.49,;-.3,-10.94,;1.03,-10.17,;2.36,-10.94,;3.81,-7.37,;2.48,-6.6,;1.38,-7.68,;2.08,-5.11,;.59,-4.71,;-.74,-3.9,;-1.3,-2.5,;-2.93,-2.64,;-4.05,-3.69,;-3.49,-5.11,;-2.22,-5.75,;-1.14,-4.72,;-1.72,-3.41,;-1.88,-5.02,;6.49,-8.9,;6.49,-10.44,;8.03,-8.9,;8.8,-7.57,;10.34,-7.57,;11.11,-6.23,;10.32,-4.91,;11.09,-3.58,;12.63,-3.57,;13.42,-4.91,;12.65,-6.24,;8.8,-10.23,;10.34,-10.23,;11.11,-11.56,;11.11,-8.9,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-26-19-34-28-10-6-5-9-27(26)28,31(39)36(20-29(37)38)12-11-21-7-3-2-4-8-21)35-32(40)41-30-24-14-22-13-23(16-24)17-25(30)15-22/h2-10,19,22-25,30,34H,11-18,20H2,1H3,(H,35,40)(H,37,38)/t22-,23+,24-,25+,30?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in rat cortex by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045806

(CHEMBL320399 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1ccc(Br)cc1)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:19.20,17.24,15.16,wD:21.23,TLB:13:14:16:19.23.18,THB:20:21:16:19.23.18,20:19:14.21.22:16,(17.3,-9.45,;15.98,-10.23,;14.64,-11,;14.66,-12.56,;15.99,-13.31,;16,-14.85,;14.67,-15.63,;14.67,-17.17,;13.33,-14.87,;13.31,-13.34,;14.47,-9.82,;13.12,-9.05,;12.04,-10.13,;12.74,-7.56,;11.25,-7.15,;9.5,-7.16,;8.43,-8.19,;7.15,-7.56,;6.6,-6.15,;7.72,-5.1,;9.35,-4.94,;9.92,-6.36,;8.77,-7.47,;8.94,-5.87,;17.31,-11,;17.3,-12.53,;18.85,-10.99,;19.63,-9.66,;21.17,-9.66,;21.94,-8.31,;23.48,-8.31,;24.25,-6.99,;23.48,-5.64,;21.92,-5.66,;21.16,-6.99,;19.63,-12.33,;21.17,-12.33,;21.94,-13.66,;21.94,-10.99,)| Show InChI InChI=1S/C31H37BrN2O5/c1-31(18-21-7-9-26(32)10-8-21,29(37)34(19-27(35)36)12-11-20-5-3-2-4-6-20)33-30(38)39-28-24-14-22-13-23(16-24)17-25(28)15-22/h2-10,22-25,28H,11-19H2,1H3,(H,33,38)(H,35,36)/t22-,23+,24-,25+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045808
(CHEMBL432389 | [1-[2-(4-Bromo-phenyl)-ethylcarbamo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Br)cc1 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(13,-12.04,;11.67,-12.82,;10.34,-13.59,;10.34,-15.14,;11.25,-16.38,;10.36,-17.64,;8.89,-17.17,;7.54,-17.94,;6.21,-17.17,;6.21,-15.62,;7.54,-14.85,;8.87,-15.62,;10.17,-12.42,;8.83,-11.65,;7.73,-12.72,;8.43,-10.16,;6.95,-9.76,;5.2,-9.76,;4.14,-10.8,;2.85,-10.16,;2.31,-8.75,;3.41,-7.7,;5.05,-7.54,;5.62,-8.96,;4.46,-10.06,;4.64,-8.47,;13,-13.59,;13,-15.12,;14.54,-13.58,;15.31,-12.25,;16.85,-12.25,;17.62,-10.9,;16.83,-9.59,;17.6,-8.26,;19.14,-8.24,;19.91,-6.91,;19.93,-9.59,;19.16,-10.92,)| Show InChI InChI=1S/C31H36BrN3O3/c1-31(17-24-18-34-27-5-3-2-4-26(24)27,29(36)33-11-10-19-6-8-25(32)9-7-19)35-30(37)38-28-22-13-20-12-21(15-22)16-23(28)14-20/h2-9,18,20-23,28,34H,10-17H2,1H3,(H,33,36)(H,35,37)/t20-,21+,22-,23+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045802

(CHEMBL99252 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1ccc2ccccc2c1)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:22.24,20.28,18.20,wD:24.25,TLB:16:17:19:22.26.21,THB:23:24:19:22.26.21,23:22:19:17.24.25,(14.77,-13.49,;13.46,-14.28,;12.13,-15.05,;12.13,-16.6,;13.47,-17.34,;13.49,-18.89,;12.15,-19.67,;12.16,-21.2,;10.84,-21.99,;9.49,-21.22,;9.49,-19.68,;10.82,-18.91,;10.8,-17.37,;11.96,-13.86,;10.62,-13.09,;9.52,-14.18,;10.22,-11.6,;8.74,-11.2,;6.99,-11.22,;5.92,-12.25,;4.63,-11.6,;4.09,-10.2,;5.19,-9.14,;6.83,-9,;7.41,-10.41,;6.25,-11.5,;6.43,-9.91,;14.8,-15.03,;14.77,-16.57,;16.33,-15.03,;17.1,-13.7,;18.65,-13.7,;19.42,-12.36,;18.63,-11.04,;19.4,-9.7,;20.94,-9.7,;21.73,-11.04,;20.96,-12.37,;17.11,-16.36,;18.65,-16.36,;19.42,-17.7,;19.42,-15.03,)| Show InChI InChI=1S/C35H40N2O5/c1-35(21-24-11-12-27-9-5-6-10-28(27)16-24,33(40)37(22-31(38)39)14-13-23-7-3-2-4-8-23)36-34(41)42-32-29-17-25-15-26(19-29)20-30(32)18-25/h2-12,16,25-26,29-30,32H,13-15,17-22H2,1H3,(H,36,41)(H,38,39)/t25-,26+,29-,30+,32?,35? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50045800

(CHEMBL317999 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(6.49,-7.36,;5.16,-8.14,;3.83,-8.91,;3.83,-10.46,;4.74,-11.7,;3.85,-12.96,;2.38,-12.49,;1.03,-13.26,;-.3,-12.49,;-.3,-10.94,;1.03,-10.17,;2.36,-10.94,;3.81,-7.37,;2.48,-6.6,;1.38,-7.68,;2.08,-5.11,;.59,-4.71,;-.74,-3.9,;-1.3,-2.5,;-2.93,-2.64,;-4.05,-3.69,;-3.49,-5.11,;-2.22,-5.75,;-1.14,-4.72,;-1.72,-3.41,;-1.88,-5.02,;6.49,-8.9,;6.49,-10.44,;8.03,-8.9,;8.8,-7.57,;10.34,-7.57,;11.11,-6.23,;10.32,-4.91,;11.09,-3.58,;12.63,-3.57,;13.42,-4.91,;12.65,-6.24,;8.8,-10.23,;10.34,-10.23,;11.11,-11.56,;11.11,-8.9,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-26-19-34-28-10-6-5-9-27(26)28,31(39)36(20-29(37)38)12-11-21-7-3-2-4-8-21)35-32(40)41-30-24-14-22-13-23(16-24)17-25(30)15-22/h2-10,19,22-25,30,34H,11-18,20H2,1H3,(H,35,40)(H,37,38)/t22-,23+,24-,25+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in mouse brain by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045813
(CHEMBL328852 | {2-(1H-Indol-3-yl)-1-methyl-1-[2-(4...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(cc1)[N+]([O-])=O |wU:17.29,wD:21.28,19.20,23.26,TLB:25:21:24:16.17.18,15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:16.17.25:22,(14.54,-5.88,;13.21,-6.66,;11.91,-7.44,;11.91,-8.98,;12.82,-10.22,;11.91,-11.48,;10.44,-11.01,;9.11,-11.78,;7.78,-11.01,;7.78,-9.47,;9.11,-8.69,;10.44,-9.46,;11.72,-6.25,;10.39,-5.48,;9.29,-6.56,;9.99,-3.99,;8.5,-3.59,;6.75,-3.6,;5.7,-4.64,;4.42,-3.99,;3.86,-2.59,;4.98,-1.54,;6.61,-1.38,;7.17,-2.8,;6.03,-3.9,;6.19,-2.31,;14.57,-7.42,;14.54,-8.96,;16.11,-7.42,;16.85,-6.09,;18.39,-6.09,;19.16,-4.74,;20.7,-4.76,;21.47,-3.43,;20.7,-2.08,;19.16,-2.1,;18.39,-3.43,;21.47,-.75,;22.71,-1.5,;21.47,.7,)| Show InChI InChI=1S/C31H36N4O5/c1-31(17-24-18-33-27-5-3-2-4-26(24)27,29(36)32-11-10-19-6-8-25(9-7-19)35(38)39)34-30(37)40-28-22-13-20-12-21(15-22)16-23(28)14-20/h2-9,18,20-23,28,33H,10-17H2,1H3,(H,32,36)(H,34,37)/t20-,21+,22-,23+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045817
(CHEMBL319085 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1ccccc1)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:18.19,16.23,14.15,wD:20.22,TLB:12:13:15:18.22.17,THB:19:20:15:18.22.17,19:18:13.20.21:15,(13.77,-12.89,;12.44,-13.67,;11.13,-14.44,;11.14,-15.99,;12.47,-16.74,;12.48,-18.28,;11.14,-19.06,;9.81,-18.3,;9.8,-16.76,;10.95,-13.26,;9.62,-12.49,;8.52,-13.58,;9.22,-11,;7.73,-10.6,;6,-10.62,;4.92,-11.65,;3.65,-11,;3.09,-9.6,;4.21,-8.55,;5.84,-8.4,;6.4,-9.81,;5.26,-10.9,;5.42,-9.32,;13.79,-14.43,;13.77,-15.97,;15.33,-14.43,;16.08,-13.1,;17.62,-13.1,;18.39,-11.76,;19.93,-11.77,;20.7,-10.44,;19.93,-9.1,;18.39,-9.11,;17.62,-10.44,;16.1,-15.76,;17.64,-15.76,;18.41,-17.09,;18.41,-14.43,)| Show InChI InChI=1S/C31H38N2O5/c1-31(19-22-10-6-3-7-11-22,29(36)33(20-27(34)35)13-12-21-8-4-2-5-9-21)32-30(37)38-28-25-15-23-14-24(17-25)18-26(28)16-23/h2-11,23-26,28H,12-20H2,1H3,(H,32,37)(H,34,35)/t23-,24+,25-,26+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50005823
(CHEMBL291033 | [2-(1H-Indol-3-yl)-1-methyl-1-phene...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccccc1 |wU:21.23,23.26,17.19,wD:19.27,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,20:19:16:21.22.25,(13.64,-8.63,;12.89,-7.3,;11.64,-6.4,;11.8,-4.86,;10.64,-3.84,;11.27,-2.42,;12.8,-2.57,;13.93,-1.52,;15.4,-1.99,;15.74,-3.52,;14.58,-4.54,;13.13,-4.08,;12.1,-8.62,;10.56,-8.6,;9.82,-7.25,;9.79,-9.91,;8.24,-9.88,;8.24,-11.43,;7.21,-12.7,;5.8,-12.13,;4.31,-12.55,;5.51,-11.27,;5.48,-9.79,;6.85,-9.3,;5.8,-10.55,;6.83,-11.77,;14.42,-7.18,;15.09,-5.8,;15.29,-8.46,;16.82,-8.34,;17.66,-9.63,;19.21,-9.52,;19.88,-8.14,;21.42,-8.02,;22.28,-9.3,;21.61,-10.69,;20.08,-10.78,)| Show InChI InChI=1S/C31H37N3O3/c1-31(18-25-19-33-27-10-6-5-9-26(25)27,29(35)32-12-11-20-7-3-2-4-8-20)34-30(36)37-28-23-14-21-13-22(16-23)17-24(28)15-21/h2-10,19,21-24,28,33H,11-18H2,1H3,(H,32,35)(H,34,36)/t21-,22+,23-,24+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045818

(CHEMBL95115 | [2-(1H-Indol-3-yl)-1-methyl-1-(2-nap...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1cccc2ccccc12 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(9.96,-9.54,;8.63,-10.31,;7.31,-11.09,;7.32,-12.64,;8.23,-13.87,;7.32,-15.13,;5.85,-14.67,;4.51,-15.44,;3.18,-14.67,;3.18,-13.13,;4.51,-12.34,;5.85,-13.11,;7.13,-9.91,;5.8,-9.14,;4.7,-10.21,;5.4,-7.65,;3.91,-7.25,;2.17,-7.25,;1.1,-8.29,;-.18,-7.65,;-.74,-6.24,;.38,-5.19,;2.02,-5.03,;2.58,-6.45,;1.43,-7.55,;1.6,-5.96,;9.97,-11.08,;9.96,-12.62,;11.51,-11.08,;12.27,-9.74,;13.81,-9.74,;14.58,-8.39,;13.81,-7.08,;14.57,-5.75,;16.12,-5.73,;16.89,-7.08,;18.41,-7.08,;19.18,-8.39,;18.41,-9.72,;16.89,-9.72,;16.12,-8.39,)| Show InChI InChI=1S/C35H39N3O3/c1-35(20-28-21-37-31-12-5-4-11-30(28)31,33(39)36-14-13-25-9-6-8-24-7-2-3-10-29(24)25)38-34(40)41-32-26-16-22-15-23(18-26)19-27(32)17-22/h2-12,21-23,26-27,32,37H,13-20H2,1H3,(H,36,39)(H,38,40)/t22-,23+,26-,27+,32?,35? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045804
(CHEMBL329088 | {2-(1H-Indol-3-yl)-1-[2-(4-methoxy-...)Show SMILES COc1ccc(CCNC(=O)C(C)(Cc2c[nH]c3ccccc23)NC(=O)OC2[C@H]3C[C@@H]4C[C@@H](C[C@H]2C4)C3)cc1 |wU:32.34,30.38,28.30,wD:34.37,TLB:26:27:29:32.36.31,THB:33:34:29:32.36.31,33:32:27.34.35:29,(21.77,-7.66,;20.21,-7.66,;19.46,-9.01,;20.24,-10.34,;19.47,-11.67,;17.93,-11.67,;17.16,-13,;15.62,-13,;14.85,-14.33,;13.31,-14.33,;13.31,-15.87,;11.98,-13.58,;13.31,-12.79,;10.65,-14.35,;10.66,-15.9,;11.57,-17.13,;10.67,-18.39,;9.2,-17.93,;7.85,-18.7,;6.52,-17.93,;6.52,-16.38,;7.85,-15.61,;9.19,-16.38,;10.48,-13.17,;9.15,-12.4,;8.05,-13.49,;8.75,-10.9,;7.26,-10.51,;5.51,-10.52,;4.44,-11.56,;3.17,-10.9,;2.62,-9.5,;3.73,-8.45,;5.37,-8.29,;5.93,-9.71,;4.78,-10.81,;4.95,-9.22,;17.15,-10.34,;17.9,-9.01,)| Show InChI InChI=1S/C32H39N3O4/c1-32(18-25-19-34-28-6-4-3-5-27(25)28,30(36)33-12-11-20-7-9-26(38-2)10-8-20)35-31(37)39-29-23-14-21-13-22(16-23)17-24(29)15-21/h3-10,19,21-24,29,34H,11-18H2,1-2H3,(H,33,36)(H,35,37)/t21-,22+,23-,24+,29?,32? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045815

(CHEMBL98516 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1cccc2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:22.24,20.28,18.20,wD:24.27,TLB:16:17:19:22.26.21,THB:23:24:19:22.26.21,23:22:19:17.24.25,(13.85,-9.4,;12.53,-10.18,;11.2,-10.95,;11.21,-12.51,;12.54,-13.27,;12.56,-14.8,;11.22,-15.58,;9.87,-14.82,;8.57,-15.6,;7.23,-14.85,;7.21,-13.3,;8.54,-12.53,;9.87,-13.29,;11.02,-9.78,;9.69,-9.01,;8.59,-10.09,;9.29,-7.51,;7.79,-7.12,;6.06,-7.12,;4.98,-8.16,;3.7,-7.51,;3.14,-6.1,;4.26,-5.05,;5.9,-4.89,;6.46,-6.31,;5.32,-7.42,;5.48,-5.82,;13.87,-10.95,;13.85,-12.49,;15.41,-10.94,;16.18,-9.61,;17.72,-9.61,;18.49,-8.26,;17.71,-6.94,;18.48,-5.61,;20.02,-5.6,;20.81,-6.94,;20.02,-8.28,;16.19,-12.28,;17.73,-12.28,;18.5,-13.62,;18.49,-10.94,)| Show InChI InChI=1S/C35H40N2O5/c1-35(21-27-12-7-11-26-10-5-6-13-30(26)27,33(40)37(22-31(38)39)15-14-23-8-3-2-4-9-23)36-34(41)42-32-28-17-24-16-25(19-28)20-29(32)18-24/h2-13,24-25,28-29,32H,14-22H2,1H3,(H,36,41)(H,38,39)/t24-,25+,28-,29+,32?,35? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045814
(CHEMBL95793 | [2-(1H-Indol-3-yl)-1-methyl-1-(3-phe...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCCc1ccccc1 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(10.65,-12.14,;9.32,-12.93,;7.99,-13.7,;8,-15.24,;8.91,-16.48,;8.01,-17.74,;6.54,-17.27,;5.19,-18.04,;3.86,-17.27,;3.86,-15.73,;5.19,-14.96,;6.53,-15.73,;7.82,-12.51,;6.49,-11.74,;5.39,-12.83,;6.09,-10.25,;4.6,-9.85,;2.85,-9.87,;1.78,-10.9,;.51,-10.25,;-.04,-8.85,;1.07,-7.8,;2.71,-7.65,;3.27,-9.06,;2.12,-10.16,;2.29,-8.57,;10.65,-13.68,;10.65,-15.22,;12.19,-13.68,;12.96,-12.35,;14.5,-12.35,;15.27,-11.01,;16.81,-11.01,;17.55,-9.68,;19.09,-9.67,;19.86,-11.01,;19.11,-12.35,;17.57,-12.35,)| Show InChI InChI=1S/C32H39N3O3/c1-32(19-26-20-34-28-12-6-5-11-27(26)28,30(36)33-13-7-10-21-8-3-2-4-9-21)35-31(37)38-29-24-15-22-14-23(17-24)18-25(29)16-22/h2-6,8-9,11-12,20,22-25,29,34H,7,10,13-19H2,1H3,(H,33,36)(H,35,37)/t22-,23+,24-,25+,29?,32? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045809

(CHEMBL316462 | [1-[2-(3,4-Dichloro-phenyl)-ethylca...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Cl)c(Cl)c1 |wU:21.23,19.27,17.19,wD:23.24,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,(13.49,-10.17,;12.16,-10.95,;10.83,-11.72,;10.83,-13.28,;11.74,-14.51,;10.85,-15.77,;9.38,-15.31,;8.03,-16.08,;6.7,-15.31,;6.7,-13.75,;8.03,-12.98,;9.36,-13.75,;10.66,-10.55,;9.32,-9.78,;8.22,-10.86,;8.92,-8.29,;7.44,-7.89,;5.69,-7.89,;4.63,-8.94,;3.34,-8.29,;2.8,-6.88,;3.9,-5.83,;5.54,-5.68,;6.11,-7.09,;4.95,-8.2,;5.13,-6.6,;13.49,-11.72,;13.49,-13.25,;15.03,-11.71,;15.8,-10.38,;17.34,-10.38,;18.11,-9.04,;17.32,-7.71,;18.09,-6.39,;19.63,-6.38,;20.4,-5.05,;20.42,-7.71,;21.96,-7.71,;19.63,-9.05,)| Show InChI InChI=1S/C31H35Cl2N3O3/c1-31(16-23-17-35-27-5-3-2-4-24(23)27,29(37)34-9-8-18-6-7-25(32)26(33)15-18)36-30(38)39-28-21-11-19-10-20(13-21)14-22(28)12-19/h2-7,15,17,19-22,28,35H,8-14,16H2,1H3,(H,34,37)(H,36,38)/t19-,20+,21-,22+,28?,31? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045801
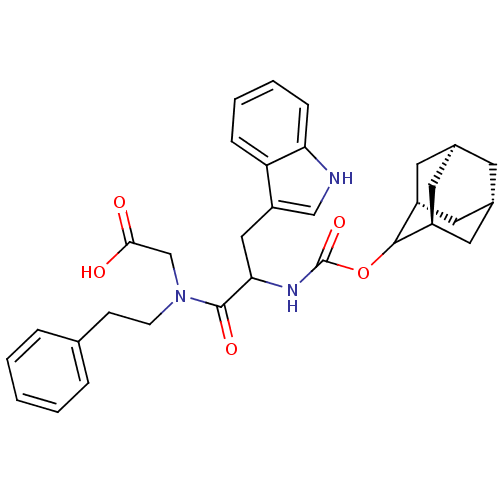
(CHEMBL95461 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES OC(=O)CN(CCc1ccccc1)C(=O)C(Cc1c[nH]c2ccccc12)NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2 |wU:37.39,wD:35.38,33.42,31.44,TLB:29:30:32:35.39.34,THB:38:33:30.37.36:39,36:37:32:35.39.34,36:35:30.37.38:32,(10.53,-4,;9.76,-2.67,;10.53,-1.33,;8.22,-2.67,;7.45,-1.34,;8.22,,;9.76,,;10.53,1.35,;12.07,1.33,;12.84,2.66,;12.06,4.01,;10.51,4.01,;9.75,2.66,;5.91,-1.34,;5.9,-2.88,;4.58,-.57,;3.25,-1.35,;3.27,-2.89,;4.18,-4.13,;3.27,-5.39,;1.8,-4.92,;.45,-5.69,;-.88,-4.92,;-.88,-3.38,;.45,-2.6,;1.8,-3.37,;3.09,-.17,;1.75,.6,;.65,-.47,;1.35,2.09,;-.13,2.49,;-1.46,3.29,;-2.03,4.71,;-3.68,4.55,;-4.78,3.5,;-4.22,2.09,;-2.96,1.45,;-1.88,2.49,;-2.44,3.78,;-2.61,2.19,)| Show InChI InChI=1S/C32H37N3O5/c36-29(37)19-35(11-10-20-6-2-1-3-7-20)31(38)28(17-25-18-33-27-9-5-4-8-26(25)27)34-32(39)40-30-23-13-21-12-22(15-23)16-24(30)14-21/h1-9,18,21-24,28,30,33H,10-17,19H2,(H,34,39)(H,36,37)/t21-,22+,23-,24+,28?,30? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045812
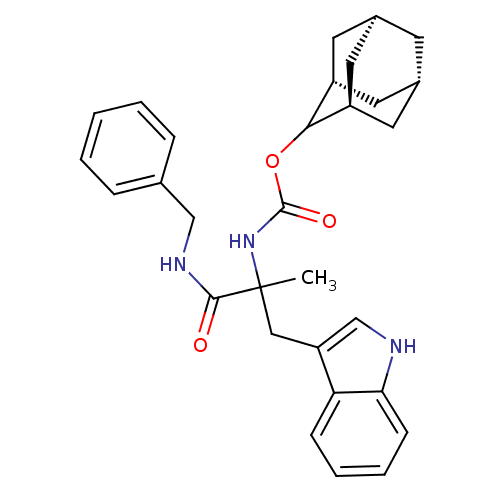
(CHEMBL99076 | [1-Benzylcarbamoyl-2-(1H-indol-3-yl)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCc1ccccc1 |wU:21.28,19.20,23.24,wD:17.19,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(19.72,-14.36,;18.39,-15.14,;17.06,-15.91,;17.08,-17.46,;17.99,-18.7,;17.09,-19.96,;15.62,-19.49,;14.26,-20.26,;12.93,-19.49,;12.93,-17.94,;14.26,-17.17,;15.61,-17.94,;16.9,-14.73,;15.56,-13.96,;14.47,-15.05,;15.17,-12.47,;13.68,-12.07,;12.35,-11.28,;11.78,-9.87,;10.15,-10.02,;9.04,-11.07,;9.59,-12.47,;10.86,-13.12,;11.93,-12.09,;11.37,-10.79,;11.2,-12.37,;19.72,-15.9,;19.72,-17.44,;21.26,-15.9,;22.03,-14.57,;23.57,-14.57,;24.34,-13.24,;25.88,-13.21,;26.65,-14.56,;25.88,-15.9,;24.34,-15.9,)| Show InChI InChI=1S/C30H35N3O3/c1-30(28(34)32-17-19-7-3-2-4-8-19,16-24-18-31-26-10-6-5-9-25(24)26)33-29(35)36-27-22-12-20-11-21(14-22)15-23(27)13-20/h2-10,18,20-23,27,31H,11-17H2,1H3,(H,32,34)(H,33,35)/t20-,21+,22-,23+,27?,30? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045819
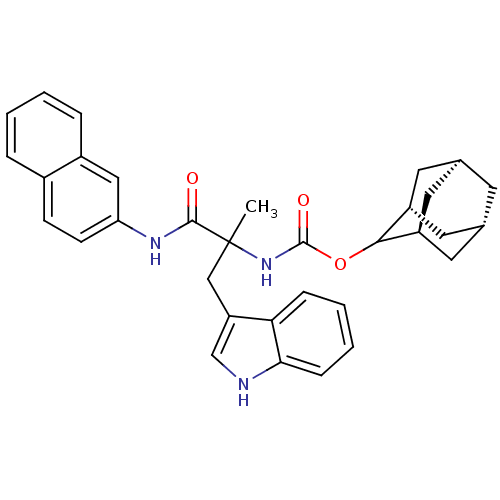
(CHEMBL95689 | [2-(1H-Indol-3-yl)-1-methyl-1-(napht...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)Nc1ccc2ccccc2c1 |wU:23.24,wD:21.23,19.27,17.19,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,(13.7,-11.7,;12.37,-12.49,;11.04,-13.26,;11.04,-14.81,;11.96,-16.05,;11.06,-17.31,;9.59,-16.84,;8.24,-17.61,;6.91,-16.84,;6.91,-15.29,;8.24,-14.52,;9.57,-15.29,;10.87,-12.07,;9.52,-11.3,;8.43,-12.39,;9.13,-9.8,;7.65,-9.41,;6.31,-8.61,;5.74,-7.2,;4.1,-7.35,;2.99,-8.4,;3.54,-9.8,;4.82,-10.46,;5.89,-9.42,;5.33,-8.12,;5.15,-9.71,;13.71,-13.24,;13.7,-14.79,;15.25,-13.24,;16.02,-11.91,;15.24,-10.58,;16,-9.24,;17.56,-9.24,;18.32,-7.91,;19.85,-7.89,;20.63,-9.24,;19.86,-10.57,;18.33,-10.57,;17.56,-11.91,)| Show InChI InChI=1S/C33H35N3O3/c1-33(18-26-19-34-29-9-5-4-8-28(26)29,31(37)35-27-11-10-22-6-2-3-7-23(22)17-27)36-32(38)39-30-24-13-20-12-21(15-24)16-25(30)14-20/h2-11,17,19-21,24-25,30,34H,12-16,18H2,1H3,(H,35,37)(H,36,38)/t20-,21+,24-,25+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 402 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045799

(CHEMBL318238 | [2-(1H-Indol-3-yl)-1-methyl-1-pheny...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)Nc1ccccc1 |wU:23.24,wD:21.23,19.27,17.19,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,(14.71,-10.97,;13.38,-11.76,;12.05,-12.53,;12.06,-14.08,;12.97,-15.31,;12.07,-16.57,;10.6,-16.11,;9.25,-16.88,;7.92,-16.11,;7.92,-14.56,;9.25,-13.79,;10.59,-14.56,;11.88,-11.35,;10.55,-10.58,;9.45,-11.67,;10.15,-9.08,;8.66,-8.69,;7.33,-7.89,;6.77,-6.49,;5.13,-6.63,;4.02,-7.68,;4.56,-9.08,;5.84,-9.74,;6.91,-8.7,;6.35,-7.4,;6.18,-8.99,;14.71,-12.51,;14.71,-14.05,;16.25,-12.51,;17.02,-11.18,;16.25,-9.85,;17.01,-8.52,;18.56,-8.52,;19.33,-9.85,;18.56,-11.18,)| Show InChI InChI=1S/C29H33N3O3/c1-29(27(33)31-23-7-3-2-4-8-23,16-22-17-30-25-10-6-5-9-24(22)25)32-28(34)35-26-20-12-18-11-19(14-20)15-21(26)13-18/h2-10,17-21,26,30H,11-16H2,1H3,(H,31,33)(H,32,34)/t18-,19+,20-,21+,26?,29? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50045800

(CHEMBL317999 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(6.49,-7.36,;5.16,-8.14,;3.83,-8.91,;3.83,-10.46,;4.74,-11.7,;3.85,-12.96,;2.38,-12.49,;1.03,-13.26,;-.3,-12.49,;-.3,-10.94,;1.03,-10.17,;2.36,-10.94,;3.81,-7.37,;2.48,-6.6,;1.38,-7.68,;2.08,-5.11,;.59,-4.71,;-.74,-3.9,;-1.3,-2.5,;-2.93,-2.64,;-4.05,-3.69,;-3.49,-5.11,;-2.22,-5.75,;-1.14,-4.72,;-1.72,-3.41,;-1.88,-5.02,;6.49,-8.9,;6.49,-10.44,;8.03,-8.9,;8.8,-7.57,;10.34,-7.57,;11.11,-6.23,;10.32,-4.91,;11.09,-3.58,;12.63,-3.57,;13.42,-4.91,;12.65,-6.24,;8.8,-10.23,;10.34,-10.23,;11.11,-11.56,;11.11,-8.9,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-26-19-34-28-10-6-5-9-27(26)28,31(39)36(20-29(37)38)12-11-21-7-3-2-4-8-21)35-32(40)41-30-24-14-22-13-23(16-24)17-25(30)15-22/h2-10,19,22-25,30,34H,11-18,20H2,1H3,(H,35,40)(H,37,38)/t22-,23+,24-,25+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 531 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type A receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045821

(CHEMBL320823 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CCOC(=O)CN(CCc1ccccc1)C(=O)C(C)(Cc1c[nH]c2ccccc12)NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2 |wU:40.42,wD:38.41,36.45,34.47,TLB:32:33:35:38.42.37,THB:41:36:33.40.39:42,39:40:35:38.42.37,39:38:33.40.41:35,(19.72,-14.24,;18.95,-12.91,;17.41,-12.91,;16.64,-11.58,;17.41,-10.24,;15.1,-11.58,;14.33,-10.25,;15.1,-8.91,;16.64,-8.91,;17.41,-7.57,;18.93,-7.58,;19.72,-6.25,;18.93,-4.91,;17.39,-4.92,;16.62,-6.25,;12.79,-10.25,;12.79,-11.79,;11.46,-9.48,;12.79,-8.71,;10.13,-10.27,;10.13,-11.81,;11.04,-13.04,;10.15,-14.3,;8.68,-13.84,;7.33,-14.61,;6,-13.84,;6,-12.3,;7.33,-11.51,;8.66,-12.28,;9.95,-9.08,;8.62,-8.31,;7.52,-9.39,;8.22,-6.82,;6.73,-6.42,;5.4,-5.62,;4.84,-4.21,;3.2,-4.36,;2.08,-5.41,;2.64,-6.82,;3.92,-7.47,;4.99,-6.42,;4.42,-5.13,;4.25,-6.73,)| Show InChI InChI=1S/C35H43N3O5/c1-3-42-31(39)22-38(14-13-23-9-5-4-6-10-23)33(40)35(2,20-28-21-36-30-12-8-7-11-29(28)30)37-34(41)43-32-26-16-24-15-25(18-26)19-27(32)17-24/h4-12,21,24-27,32,36H,3,13-20,22H2,1-2H3,(H,37,41)/t24-,25+,26-,27+,32?,35? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045820
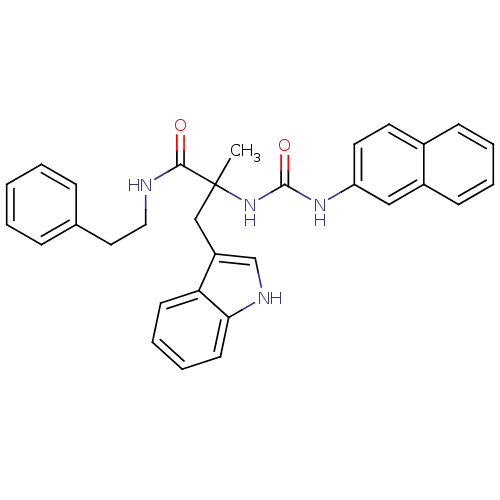
(3-(1H-Indol-3-yl)-2-methyl-2-(3-naphthalen-2-yl-ur...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc2ccccc2c1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H30N4O2/c1-31(20-25-21-33-28-14-8-7-13-27(25)28,29(36)32-18-17-22-9-3-2-4-10-22)35-30(37)34-26-16-15-23-11-5-6-12-24(23)19-26/h2-16,19,21,33H,17-18,20H2,1H3,(H,32,36)(H2,34,35,37) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 769 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045811

(CHEMBL96036 | [2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCC(O)=O |wU:21.28,19.20,23.24,wD:17.19,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(14.82,-10.14,;13.5,-10.93,;12.18,-11.69,;12.19,-13.24,;13.1,-14.47,;12.19,-15.73,;10.72,-15.27,;9.39,-16.04,;8.06,-15.27,;8.06,-13.72,;9.39,-12.95,;10.72,-13.72,;12.01,-10.51,;10.68,-9.75,;9.58,-10.83,;10.28,-8.25,;8.79,-7.86,;7.46,-7.07,;6.9,-5.66,;5.27,-5.81,;4.15,-6.86,;4.71,-8.25,;5.99,-8.91,;7.06,-7.87,;6.48,-6.58,;6.32,-8.16,;14.84,-11.68,;14.82,-13.22,;16.38,-11.68,;17.15,-13.01,;18.67,-13.01,;19.45,-14.33,;19.76,-11.91,)| Show InChI InChI=1S/C25H31N3O5/c1-25(23(31)27-13-21(29)30,11-18-12-26-20-5-3-2-4-19(18)20)28-24(32)33-22-16-7-14-6-15(9-16)10-17(22)8-14/h2-5,12,14-17,22,26H,6-11,13H2,1H3,(H,27,31)(H,28,32)(H,29,30)/t14-,15+,16-,17+,22?,25? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 986 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045816

(CHEMBL319880 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:17.19,wD:21.28,19.20,23.26,TLB:25:21:24:16.17.18,15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(25.85,-.15,;24.53,-.92,;23.2,-1.7,;23.22,-3.24,;24.13,-4.48,;23.22,-5.74,;21.75,-5.27,;20.4,-6.04,;19.07,-5.27,;19.07,-3.73,;20.4,-2.95,;21.75,-3.72,;23.03,-.52,;21.68,.25,;20.6,-.82,;21.3,1.74,;19.81,2.14,;18.07,2.14,;16.99,1.1,;15.73,1.74,;15.17,3.15,;16.29,4.2,;17.92,4.36,;18.49,2.94,;17.34,1.84,;17.51,3.43,;25.86,-1.69,;25.85,-3.23,;27.4,-1.69,;28.17,-.35,;29.71,-.35,;30.48,1,;29.7,2.31,;30.46,3.66,;32.01,3.66,;32.77,4.99,;32.79,2.31,;32.02,.98,;32.78,-.35,;28.17,-3.02,;29.71,-3.02,;30.48,-4.35,;30.48,-1.68,)| Show InChI InChI=1S/C33H37Cl2N3O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,37-32(42)43-30-22-11-19-10-20(13-22)14-23(30)12-19)31(41)38(18-29(39)40)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,37,42)(H,39,40)/t19-,20+,22-,23+,30?,33? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50045805

(CHEMBL318852 | N-Carboxymethyl-N-[2-(2,4-dichloro-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(C(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1Cl)CC(O)=O |wU:20.27,18.19,22.23,wD:16.28,TLB:14:15:21:18.23.19,THB:17:16:21:18.23.19,17:18:21:15.16.24,(15.87,-6.38,;14.54,-7.15,;13.21,-7.93,;13.23,-9.47,;14.14,-10.71,;13.24,-11.97,;11.77,-11.5,;10.41,-12.27,;9.08,-11.5,;9.08,-9.96,;10.41,-9.18,;11.76,-9.95,;13.04,-6.75,;11.95,-7.82,;12.65,-5.26,;11.16,-4.86,;9.83,-4.06,;9.27,-2.64,;7.64,-2.8,;6.52,-3.85,;7.08,-5.26,;8.35,-5.9,;9.41,-4.86,;8.85,-3.57,;8.69,-5.16,;15.87,-7.92,;15.87,-9.46,;17.41,-7.92,;18.18,-6.58,;19.72,-6.58,;20.49,-5.23,;19.72,-3.92,;20.47,-2.59,;22.03,-2.57,;22.78,-1.24,;22.8,-3.92,;22.03,-5.25,;22.8,-6.58,;18.18,-9.25,;19.72,-9.25,;20.49,-10.58,;20.49,-7.91,)| Show InChI InChI=1S/C33H36Cl2N2O5/c1-33(16-24-17-36-28-5-3-2-4-26(24)28,32(41)42-30-22-11-19-10-20(13-22)14-23(30)12-19)31(40)37(18-29(38)39)9-8-21-6-7-25(34)15-27(21)35/h2-7,15,17,19-20,22-23,30,36H,8-14,16,18H2,1H3,(H,38,39)/t19-,20+,22-,23+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type A receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type B receptor in mouse brain by displacement of [3H]-pBC264 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045810

(3-{[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indol...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCC(O)=O)CCc1ccccc1 |wU:23.26,wD:21.23,19.27,17.29,TLB:24:19:25:16.23.22,15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(12.3,-9.53,;10.97,-10.3,;9.66,-11.08,;9.67,-12.62,;10.58,-13.86,;9.67,-15.12,;8.2,-14.65,;6.86,-15.42,;5.53,-14.65,;5.53,-13.11,;6.86,-12.33,;8.2,-13.1,;9.48,-9.9,;8.14,-9.13,;7.05,-10.2,;7.75,-7.64,;6.26,-7.24,;4.93,-6.44,;4.37,-5.02,;2.74,-5.18,;1.62,-6.23,;2.18,-7.64,;3.45,-8.28,;4.51,-7.24,;3.95,-5.95,;3.79,-7.54,;12.32,-11.07,;12.3,-12.61,;13.86,-11.07,;14.63,-12.4,;16.17,-12.4,;16.94,-13.73,;18.48,-13.73,;16.17,-15.06,;14.61,-9.73,;16.15,-9.73,;16.92,-8.38,;16.15,-7.07,;16.92,-5.74,;18.46,-5.72,;19.23,-7.07,;18.46,-8.4,)| Show InChI InChI=1S/C34H41N3O5/c1-34(20-27-21-35-29-10-6-5-9-28(27)29,32(40)37(14-12-30(38)39)13-11-22-7-3-2-4-8-22)36-33(41)42-31-25-16-23-15-24(18-25)19-26(31)17-23/h2-10,21,23-26,31,35H,11-20H2,1H3,(H,36,41)(H,38,39)/t23-,24+,25-,26+,31?,34? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50045796

(CHEMBL99939 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(1.85,-13.1,;.54,-13.87,;-.79,-14.65,;-.79,-16.19,;.12,-17.43,;-.77,-18.69,;-2.24,-18.22,;-3.59,-18.99,;-4.92,-18.22,;-4.92,-16.68,;-3.59,-15.9,;-2.26,-16.67,;-.97,-13.47,;-2.3,-12.7,;-3.4,-13.77,;-2.7,-11.21,;-4.18,-10.81,;-5.51,-10.01,;-6.08,-8.59,;-7.72,-8.75,;-8.82,-9.8,;-8.28,-11.21,;-6.99,-11.85,;-5.93,-10.81,;-6.49,-9.52,;-6.67,-11.11,;1.87,-14.64,;1.85,-16.18,;3.41,-14.64,;4.18,-13.3,;5.72,-13.3,;6.49,-11.95,;8.03,-11.97,;8.8,-10.64,;8.01,-9.29,;8.77,-7.96,;6.47,-9.31,;5.7,-10.64,;4.18,-15.97,;5.72,-15.97,;6.49,-17.3,;6.49,-14.63,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21-,22+,23-,24+,30?,33? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards cholecystokinin type A receptor in rat pancreas by displacement of [3H]-pCCK-8 radioligand |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045800

(CHEMBL317999 | {[2-(Adamantan-2-yloxycarbonylamino...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:23.24,wD:21.23,19.27,17.29,TLB:15:16:18:21.25.20,THB:24:19:16.23.22:25,22:23:18:21.25.20,22:21:16.23.24:18,(6.49,-7.36,;5.16,-8.14,;3.83,-8.91,;3.83,-10.46,;4.74,-11.7,;3.85,-12.96,;2.38,-12.49,;1.03,-13.26,;-.3,-12.49,;-.3,-10.94,;1.03,-10.17,;2.36,-10.94,;3.81,-7.37,;2.48,-6.6,;1.38,-7.68,;2.08,-5.11,;.59,-4.71,;-.74,-3.9,;-1.3,-2.5,;-2.93,-2.64,;-4.05,-3.69,;-3.49,-5.11,;-2.22,-5.75,;-1.14,-4.72,;-1.72,-3.41,;-1.88,-5.02,;6.49,-8.9,;6.49,-10.44,;8.03,-8.9,;8.8,-7.57,;10.34,-7.57,;11.11,-6.23,;10.32,-4.91,;11.09,-3.58,;12.63,-3.57,;13.42,-4.91,;12.65,-6.24,;8.8,-10.23,;10.34,-10.23,;11.11,-11.56,;11.11,-8.9,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-26-19-34-28-10-6-5-9-27(26)28,31(39)36(20-29(37)38)12-11-21-7-3-2-4-8-21)35-32(40)41-30-24-14-22-13-23(16-24)17-25(30)15-22/h2-10,19,22-25,30,34H,11-18,20H2,1H3,(H,35,40)(H,37,38)/t22-,23+,24-,25+,30?,33? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045809

(CHEMBL316462 | [1-[2-(3,4-Dichloro-phenyl)-ethylca...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Cl)c(Cl)c1 |wU:21.23,19.27,17.19,wD:23.24,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,(13.49,-10.17,;12.16,-10.95,;10.83,-11.72,;10.83,-13.28,;11.74,-14.51,;10.85,-15.77,;9.38,-15.31,;8.03,-16.08,;6.7,-15.31,;6.7,-13.75,;8.03,-12.98,;9.36,-13.75,;10.66,-10.55,;9.32,-9.78,;8.22,-10.86,;8.92,-8.29,;7.44,-7.89,;5.69,-7.89,;4.63,-8.94,;3.34,-8.29,;2.8,-6.88,;3.9,-5.83,;5.54,-5.68,;6.11,-7.09,;4.95,-8.2,;5.13,-6.6,;13.49,-11.72,;13.49,-13.25,;15.03,-11.71,;15.8,-10.38,;17.34,-10.38,;18.11,-9.04,;17.32,-7.71,;18.09,-6.39,;19.63,-6.38,;20.4,-5.05,;20.42,-7.71,;21.96,-7.71,;19.63,-9.05,)| Show InChI InChI=1S/C31H35Cl2N3O3/c1-31(16-23-17-35-27-5-3-2-4-24(23)27,29(37)34-9-8-18-6-7-25(32)26(33)15-18)36-30(38)39-28-21-11-19-10-20(13-21)14-22(28)12-19/h2-7,15,17,19-22,28,35H,8-14,16H2,1H3,(H,34,37)(H,36,38)/t19-,20+,21-,22+,28?,31? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045803

(CHEMBL433407 | [1-[2-(4-Chloro-phenyl)-ethylcarbam...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Cl)cc1 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(14.26,-12.21,;12.93,-13,;11.6,-13.77,;11.6,-15.31,;12.51,-16.55,;11.62,-17.81,;10.15,-17.34,;8.8,-18.11,;7.47,-17.34,;7.47,-15.8,;8.8,-15.03,;10.13,-15.8,;11.43,-12.58,;10.09,-11.81,;8.99,-12.9,;9.69,-10.32,;8.21,-9.92,;6.46,-9.94,;5.4,-10.97,;4.11,-10.32,;3.57,-8.92,;4.67,-7.87,;6.31,-7.71,;6.88,-9.13,;5.72,-10.23,;5.9,-8.64,;14.26,-13.75,;14.26,-15.29,;15.8,-13.75,;16.57,-12.42,;18.11,-12.42,;18.88,-11.08,;18.09,-9.76,;18.86,-8.43,;20.4,-8.41,;21.17,-7.08,;21.19,-9.76,;20.42,-11.09,)| Show InChI InChI=1S/C31H36ClN3O3/c1-31(17-24-18-34-27-5-3-2-4-26(24)27,29(36)33-11-10-19-6-8-25(32)9-7-19)35-30(37)38-28-22-13-20-12-21(15-22)16-23(28)14-20/h2-9,18,20-23,28,34H,10-17H2,1H3,(H,33,36)(H,35,37)/t20-,21+,22-,23+,28?,31? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045807
(3-(1H-Indol-3-yl)-2-methyl-N-phenethyl-2-(3-trityl...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)NC(c1ccccc1)(c1ccccc1)c1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C40H38N4O2/c1-39(28-31-29-42-36-25-15-14-24-35(31)36,37(45)41-27-26-30-16-6-2-7-17-30)43-38(46)44-40(32-18-8-3-9-19-32,33-20-10-4-11-21-33)34-22-12-5-13-23-34/h2-25,29,42H,26-28H2,1H3,(H,41,45)(H2,43,44,46) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50005823

(CHEMBL291033 | [2-(1H-Indol-3-yl)-1-methyl-1-phene...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccccc1 |wU:21.23,23.26,17.19,wD:19.27,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,20:19:16:21.22.25,(13.64,-8.63,;12.89,-7.3,;11.64,-6.4,;11.8,-4.86,;10.64,-3.84,;11.27,-2.42,;12.8,-2.57,;13.93,-1.52,;15.4,-1.99,;15.74,-3.52,;14.58,-4.54,;13.13,-4.08,;12.1,-8.62,;10.56,-8.6,;9.82,-7.25,;9.79,-9.91,;8.24,-9.88,;8.24,-11.43,;7.21,-12.7,;5.8,-12.13,;4.31,-12.55,;5.51,-11.27,;5.48,-9.79,;6.85,-9.3,;5.8,-10.55,;6.83,-11.77,;14.42,-7.18,;15.09,-5.8,;15.29,-8.46,;16.82,-8.34,;17.66,-9.63,;19.21,-9.52,;19.88,-8.14,;21.42,-8.02,;22.28,-9.3,;21.61,-10.69,;20.08,-10.78,)| Show InChI InChI=1S/C31H37N3O3/c1-31(18-25-19-33-27-10-6-5-9-26(25)27,29(35)32-12-11-20-7-3-2-4-8-20)34-30(36)37-28-23-14-21-13-22(16-23)17-24(28)15-21/h2-10,19,21-24,28,33H,11-18H2,1H3,(H,32,35)(H,34,36)/t21-,22+,23-,24+,28?,31? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045815

(CHEMBL98516 | {[2-(Adamantan-2-yloxycarbonylamino)...)Show SMILES CC(Cc1cccc2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N(CCc1ccccc1)CC(O)=O |wU:22.24,20.28,18.20,wD:24.27,TLB:16:17:19:22.26.21,THB:23:24:19:22.26.21,23:22:19:17.24.25,(13.85,-9.4,;12.53,-10.18,;11.2,-10.95,;11.21,-12.51,;12.54,-13.27,;12.56,-14.8,;11.22,-15.58,;9.87,-14.82,;8.57,-15.6,;7.23,-14.85,;7.21,-13.3,;8.54,-12.53,;9.87,-13.29,;11.02,-9.78,;9.69,-9.01,;8.59,-10.09,;9.29,-7.51,;7.79,-7.12,;6.06,-7.12,;4.98,-8.16,;3.7,-7.51,;3.14,-6.1,;4.26,-5.05,;5.9,-4.89,;6.46,-6.31,;5.32,-7.42,;5.48,-5.82,;13.87,-10.95,;13.85,-12.49,;15.41,-10.94,;16.18,-9.61,;17.72,-9.61,;18.49,-8.26,;17.71,-6.94,;18.48,-5.61,;20.02,-5.6,;20.81,-6.94,;20.02,-8.28,;16.19,-12.28,;17.73,-12.28,;18.5,-13.62,;18.49,-10.94,)| Show InChI InChI=1S/C35H40N2O5/c1-35(21-27-12-7-11-26-10-5-6-13-30(26)27,33(40)37(22-31(38)39)15-14-23-8-3-2-4-9-23)36-34(41)42-32-28-17-24-16-25(19-28)20-29(32)18-24/h2-13,24-25,28-29,32H,14-22H2,1H3,(H,36,41)(H,38,39)/t24-,25+,28-,29+,32?,35? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50045808

(CHEMBL432389 | [1-[2-(4-Bromo-phenyl)-ethylcarbamo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NCCc1ccc(Br)cc1 |wU:21.23,19.27,17.19,wD:23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(13,-12.04,;11.67,-12.82,;10.34,-13.59,;10.34,-15.14,;11.25,-16.38,;10.36,-17.64,;8.89,-17.17,;7.54,-17.94,;6.21,-17.17,;6.21,-15.62,;7.54,-14.85,;8.87,-15.62,;10.17,-12.42,;8.83,-11.65,;7.73,-12.72,;8.43,-10.16,;6.95,-9.76,;5.2,-9.76,;4.14,-10.8,;2.85,-10.16,;2.31,-8.75,;3.41,-7.7,;5.05,-7.54,;5.62,-8.96,;4.46,-10.06,;4.64,-8.47,;13,-13.59,;13,-15.12,;14.54,-13.58,;15.31,-12.25,;16.85,-12.25,;17.62,-10.9,;16.83,-9.59,;17.6,-8.26,;19.14,-8.24,;19.91,-6.91,;19.93,-9.59,;19.16,-10.92,)| Show InChI InChI=1S/C31H36BrN3O3/c1-31(17-24-18-34-27-5-3-2-4-26(24)27,29(36)33-11-10-19-6-8-25(32)9-7-19)35-30(37)38-28-22-13-20-12-21(15-22)16-23(28)14-20/h2-9,18,20-23,28,34H,10-17H2,1H3,(H,33,36)(H,35,37)/t20-,21+,22-,23+,28?,31? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type A receptor in guinea pig pancreatic membranes |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































