

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039721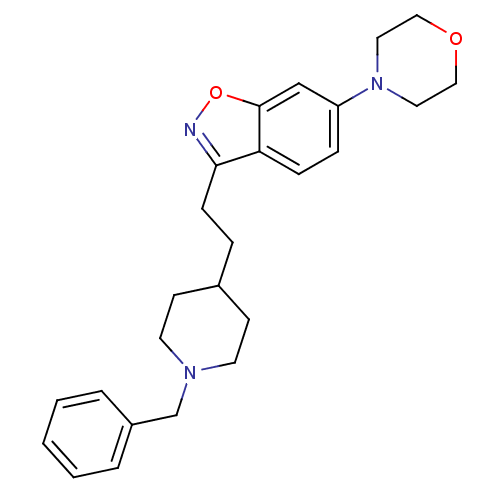 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032165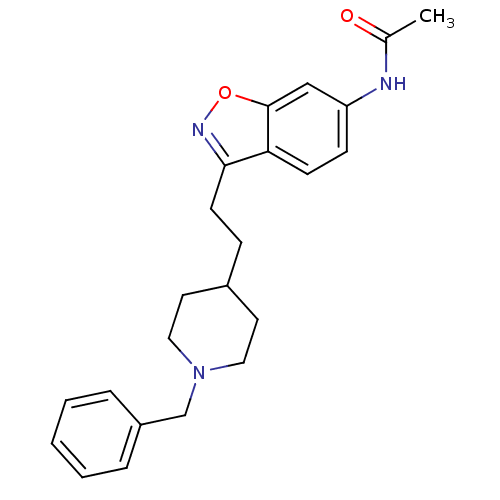 (CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039729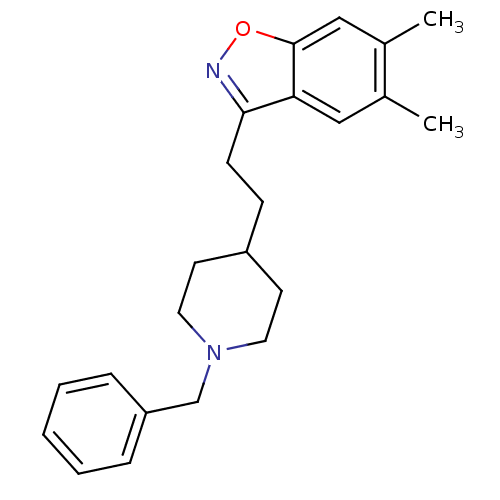 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039713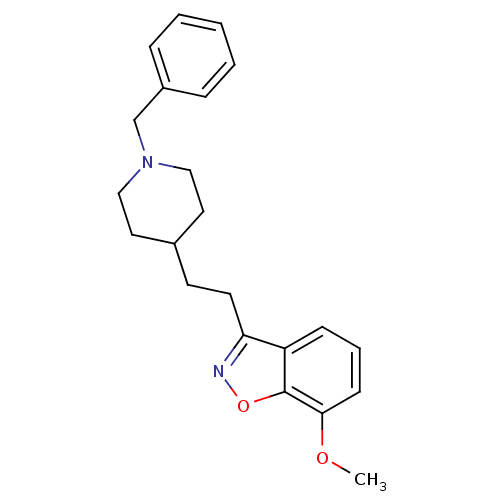 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039712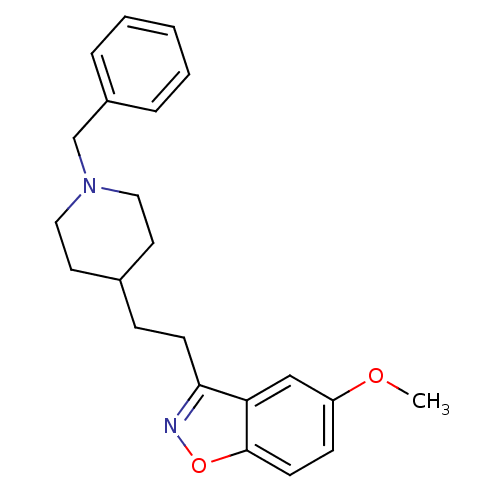 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039728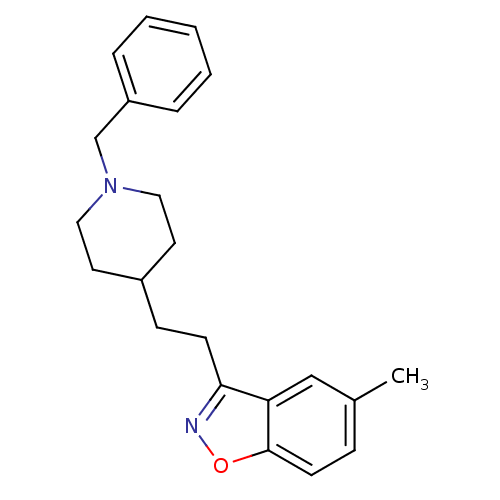 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039717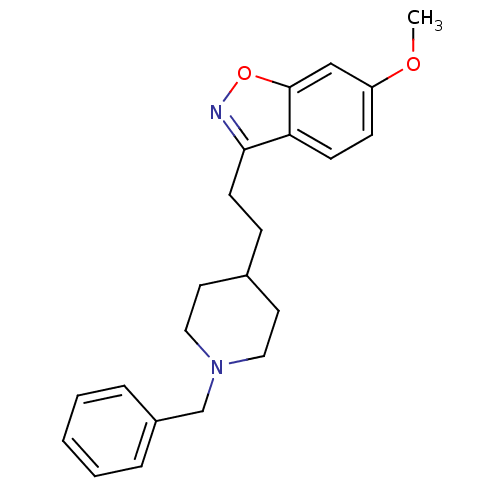 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039723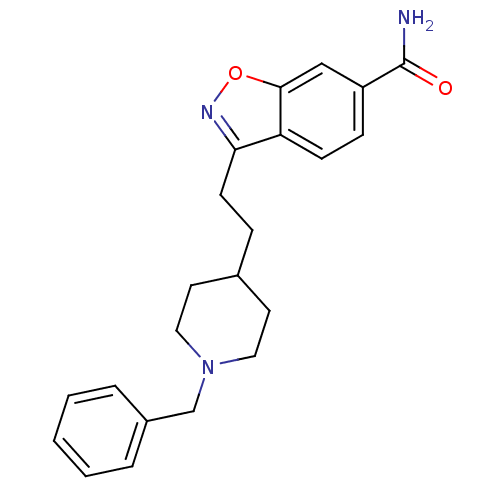 (3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039727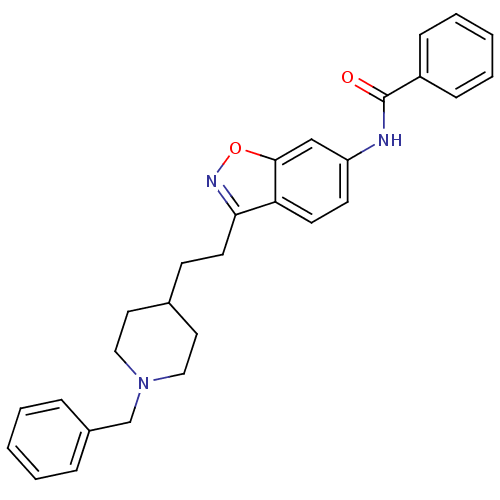 (CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039725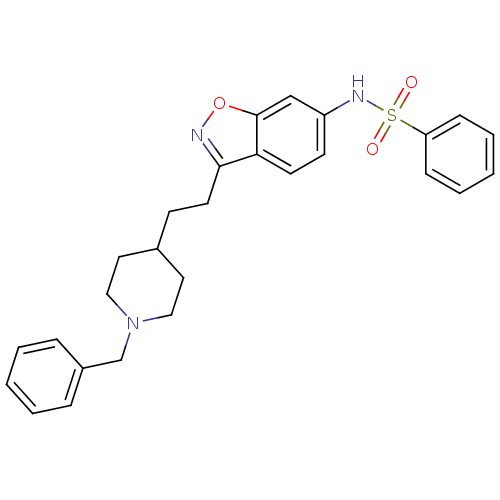 (CHEMBL92955 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039719 (3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039731 (3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039734 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-bromobenzo[d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039716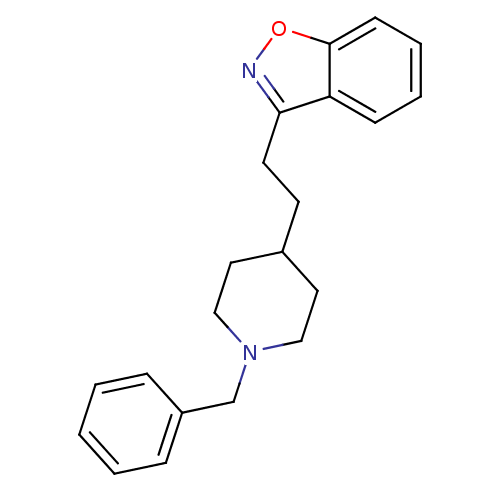 (3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039715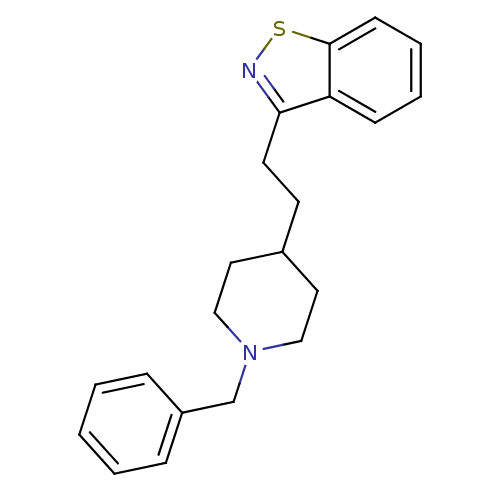 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-benzo[d]isot...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039718 (3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039720 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1H-indazole ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039726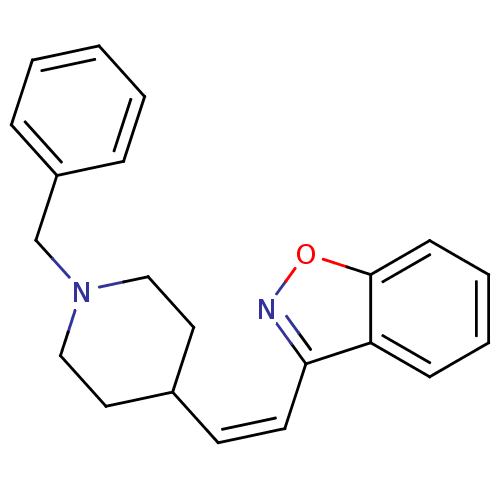 (3-[(Z)-2-(1-Benzyl-piperidin-4-yl)-vinyl]-benzo[d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039733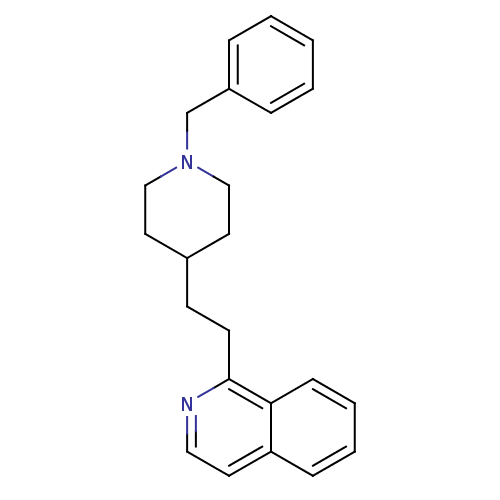 (1-(2-(1-benzylpiperidin-4-yl)ethyl)isoquinoline | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes, | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039711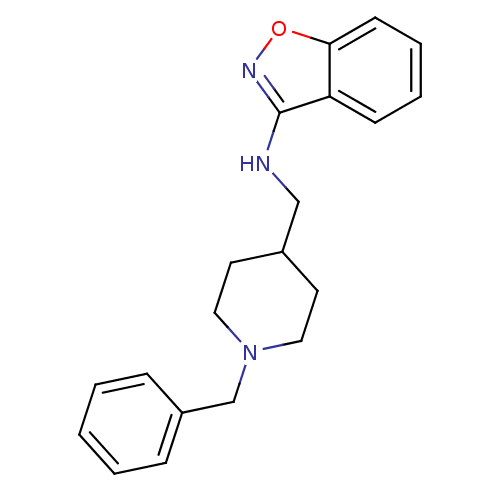 (Benzo[d]isoxazol-3-yl-(1-benzyl-piperidin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039732 (4-(2-(1-benzylpiperidin-4-yl)ethyl)quinazoline | 4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039724 (Benzo[d]isoxazol-3-yl-[2-(1-benzyl-piperidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039730 (3-(3-(1-benzylpiperidin-4-yl)propyl)benzo[d]isoxaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50039722 (3-(1-Benzyl-piperidin-4-ylmethoxy)-benzo[d]isoxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50039721 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50032165 (CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum | J Med Chem 37: 2721-34 (1994) BindingDB Entry DOI: 10.7270/Q2VT1R4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||