

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040857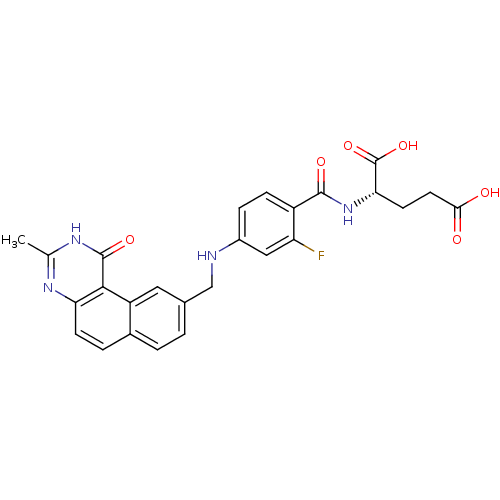 ((S)-2-{2-Fluoro-4-[(3-methyl-1-oxo-1,2-dihydro-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040861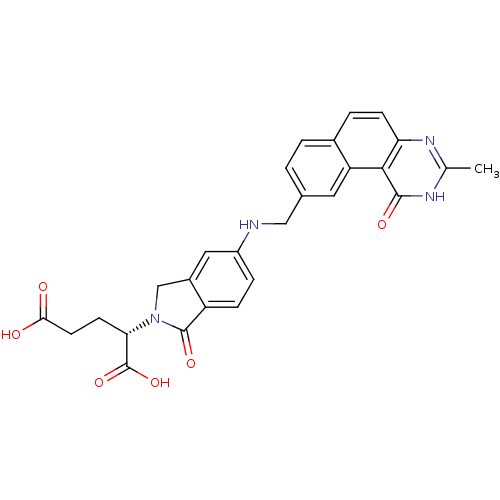 ((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040865 ((S)-2-{4-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040860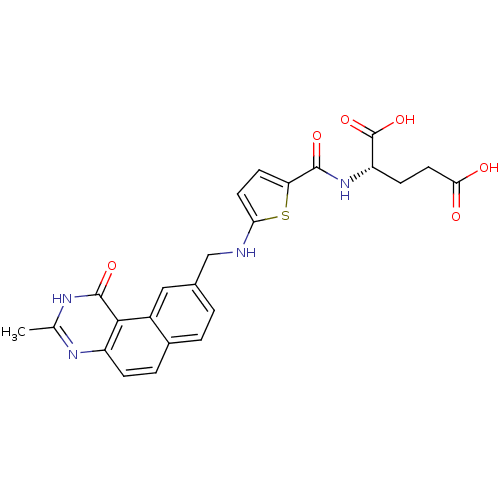 ((S)-2-({5-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040863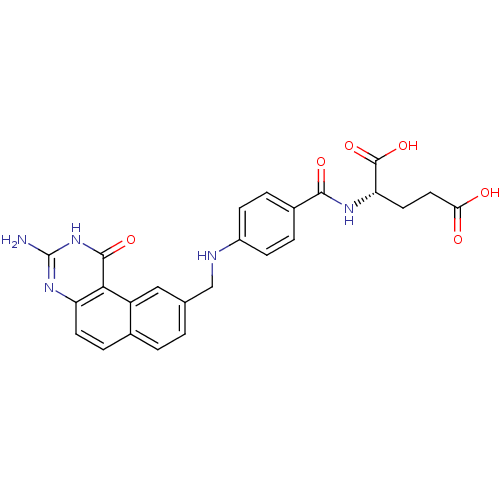 ((S)-2-{4-[(3-Amino-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040859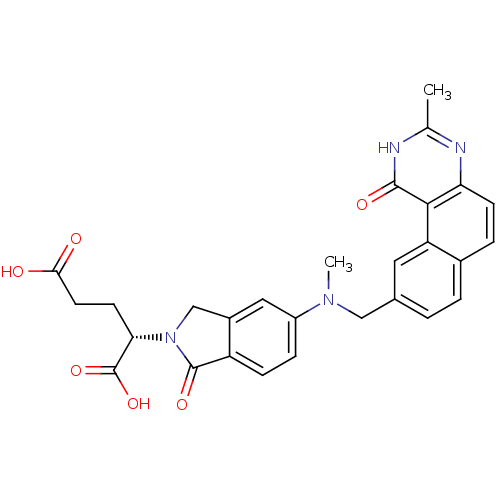 ((S)-2-{5-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040862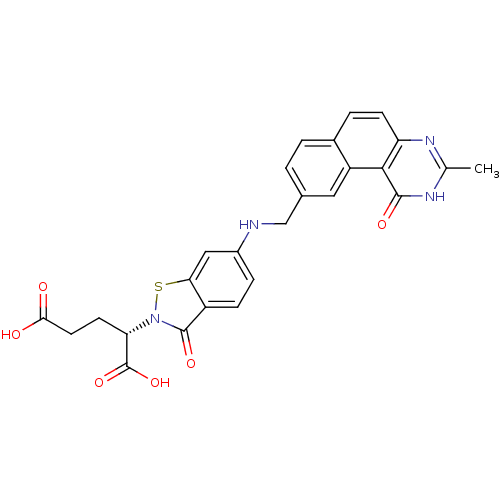 ((S)-2-{6-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040858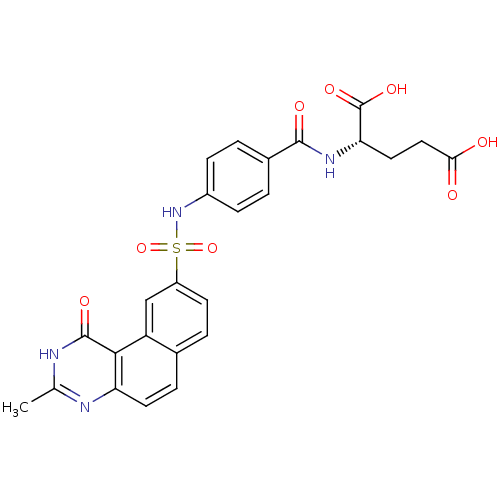 ((S)-2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040864 ((S)-2-{4-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||