 Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50043209
Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50043209 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50437405
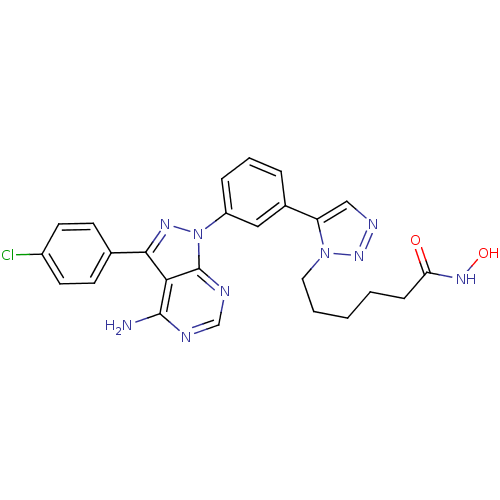
(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005012
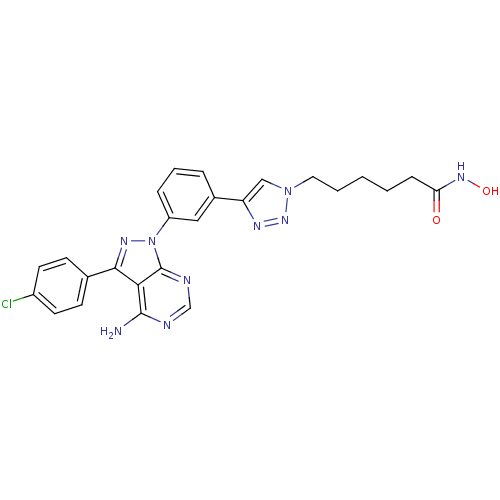
(CHEMBL2408777)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cn(CCCCCC(=O)NO)nn1 Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-34(33-30-20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005013
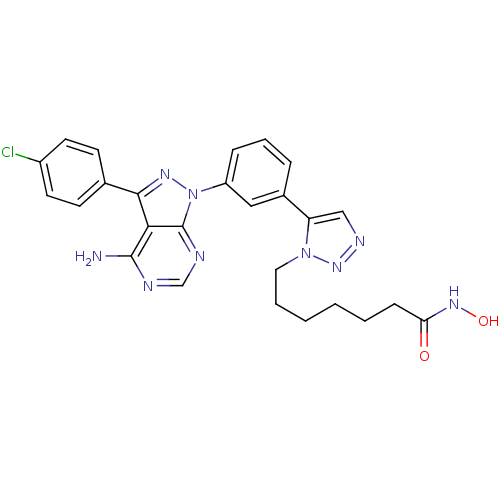
(CHEMBL2408779)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCCC(=O)NO Show InChI InChI=1S/C26H26ClN9O2/c27-19-11-9-17(10-12-19)24-23-25(28)29-16-30-26(23)36(32-24)20-7-5-6-18(14-20)21-15-31-34-35(21)13-4-2-1-3-8-22(37)33-38/h5-7,9-12,14-16,38H,1-4,8,13H2,(H,33,37)(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005015
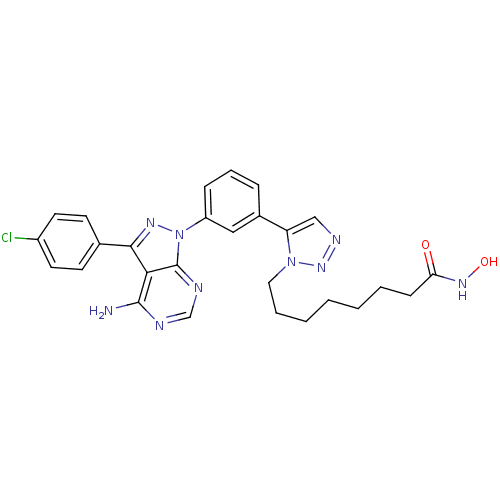
(CHEMBL2408780)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCCCC(=O)NO Show InChI InChI=1S/C27H28ClN9O2/c28-20-12-10-18(11-13-20)25-24-26(29)30-17-31-27(24)37(33-25)21-8-6-7-19(15-21)22-16-32-35-36(22)14-5-3-1-2-4-9-23(38)34-39/h6-8,10-13,15-17,39H,1-5,9,14H2,(H,34,38)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005016
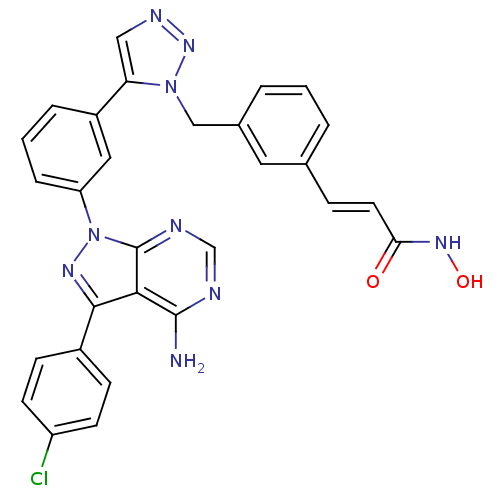
(CHEMBL2408782)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1Cc1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C29H22ClN9O2/c30-22-10-8-20(9-11-22)27-26-28(31)32-17-33-29(26)39(35-27)23-6-2-5-21(14-23)24-15-34-37-38(24)16-19-4-1-3-18(13-19)7-12-25(40)36-41/h1-15,17,41H,16H2,(H,36,40)(H2,31,32,33)/b12-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005014
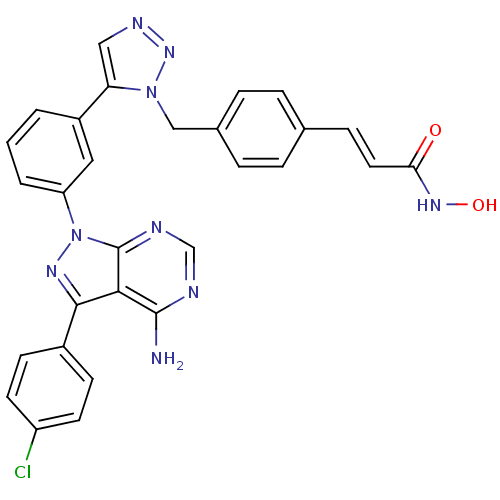
(CHEMBL2408781)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C29H22ClN9O2/c30-22-11-9-20(10-12-22)27-26-28(31)32-17-33-29(26)39(35-27)23-3-1-2-21(14-23)24-15-34-37-38(24)16-19-6-4-18(5-7-19)8-13-25(40)36-41/h1-15,17,41H,16H2,(H,36,40)(H2,31,32,33)/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Leu-Gly-Lys(Ac)AMCA as substrate after 30 mins by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005018
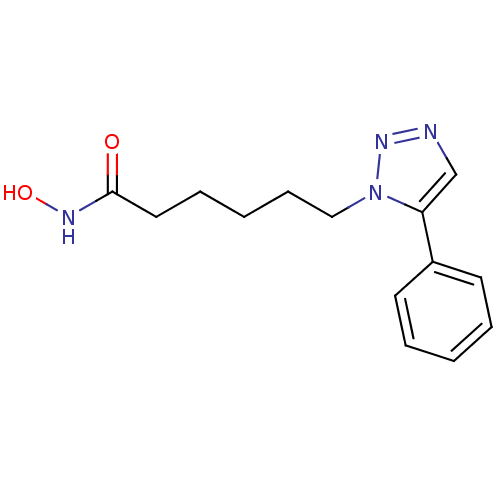
(CHEMBL2407108)Show InChI InChI=1S/C14H18N4O2/c19-14(16-20)9-5-2-6-10-18-13(11-15-17-18)12-7-3-1-4-8-12/h1,3-4,7-8,11,20H,2,5-6,9-10H2,(H,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005013

(CHEMBL2408779)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCCC(=O)NO Show InChI InChI=1S/C26H26ClN9O2/c27-19-11-9-17(10-12-19)24-23-25(28)29-16-30-26(23)36(32-24)20-7-5-6-18(14-20)21-15-31-34-35(21)13-4-2-1-3-8-22(37)33-38/h5-7,9-12,14-16,38H,1-4,8,13H2,(H,33,37)(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005012

(CHEMBL2408777)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cn(CCCCCC(=O)NO)nn1 Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-34(33-30-20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005015

(CHEMBL2408780)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCCCC(=O)NO Show InChI InChI=1S/C27H28ClN9O2/c28-20-12-10-18(11-13-20)25-24-26(29)30-17-31-27(24)37(33-25)21-8-6-7-19(15-21)22-16-32-35-36(22)14-5-3-1-2-4-9-23(38)34-39/h6-8,10-13,15-17,39H,1-5,9,14H2,(H,34,38)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HCK (unknown origin) |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005017
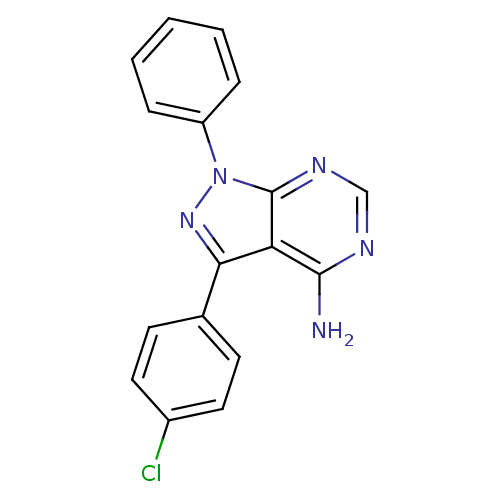
(CHEMBL2408783)Show InChI InChI=1S/C17H12ClN5/c18-12-8-6-11(7-9-12)15-14-16(19)20-10-21-17(14)23(22-15)13-4-2-1-3-5-13/h1-10H,(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005016

(CHEMBL2408782)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1Cc1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C29H22ClN9O2/c30-22-10-8-20(9-11-22)27-26-28(31)32-17-33-29(26)39(35-27)23-6-2-5-21(14-23)24-15-34-37-38(24)16-19-4-1-3-18(13-19)7-12-25(40)36-41/h1-15,17,41H,16H2,(H,36,40)(H2,31,32,33)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Abl (unknown origin) |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005014

(CHEMBL2408781)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C29H22ClN9O2/c30-22-11-9-20(10-12-22)27-26-28(31)32-17-33-29(26)39(35-27)23-3-1-2-21(14-23)24-15-34-37-38(24)16-19-6-4-18(5-7-19)8-13-25(40)36-41/h1-15,17,41H,16H2,(H,36,40)(H2,31,32,33)/b13-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) after 10 mins by fluorimetric assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005017

(CHEMBL2408783)Show InChI InChI=1S/C17H12ClN5/c18-12-8-6-11(7-9-12)15-14-16(19)20-10-21-17(14)23(22-15)13-4-2-1-3-5-13/h1-10H,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by Fluor de Lys based assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50005018

(CHEMBL2407108)Show InChI InChI=1S/C14H18N4O2/c19-14(16-20)9-5-2-6-10-18-13(11-15-17-18)12-7-3-1-4-8-12/h1,3-4,7-8,11,20H,2,5-6,9-10H2,(H,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.25E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of c-Src (unknown origin) by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHK(Ac)K(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHKK(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic peptide from p53 residues (379 to 382) (RHK(Ac)K(Ac)) as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50437405

(CHEMBL2408778)Show SMILES Nc1ncnc2n(nc(-c3ccc(Cl)cc3)c12)-c1cccc(c1)-c1cnnn1CCCCCC(=O)NO Show InChI InChI=1S/C25H24ClN9O2/c26-18-10-8-16(9-11-18)23-22-24(27)28-15-29-25(22)35(31-23)19-6-4-5-17(13-19)20-14-30-33-34(20)12-3-1-2-7-21(36)32-37/h4-6,8-11,13-15,37H,1-3,7,12H2,(H,32,36)(H2,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC9 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of HDAC9 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 779-783 (2013)
Article DOI: 10.1021/ml400175d
BindingDB Entry DOI: 10.7270/Q2CZ38KR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data