 Found 70 hits Enz. Inhib. hit(s) with all data for entry = 50006429
Found 70 hits Enz. Inhib. hit(s) with all data for entry = 50006429 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049038
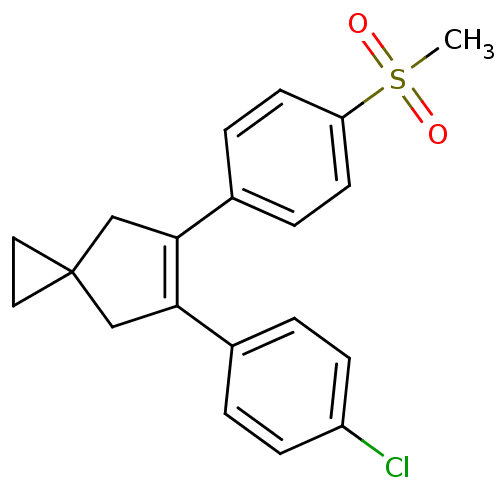
(5-(4-Chloro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C20H19ClO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049011
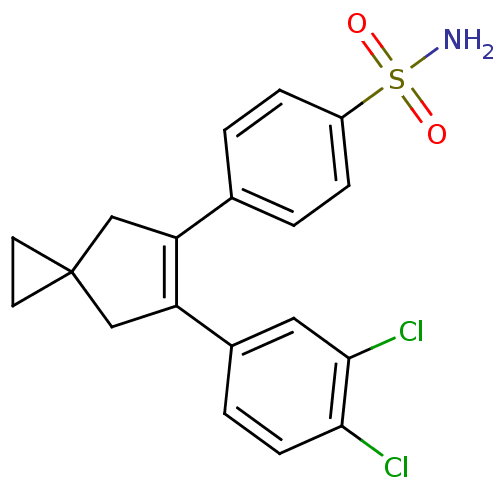
(4-[6-(3,4-Dichloro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17Cl2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049042

(4-[6-(4-Trifluoromethyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C20H18F3NO2S/c21-20(22,23)15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)27(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049025
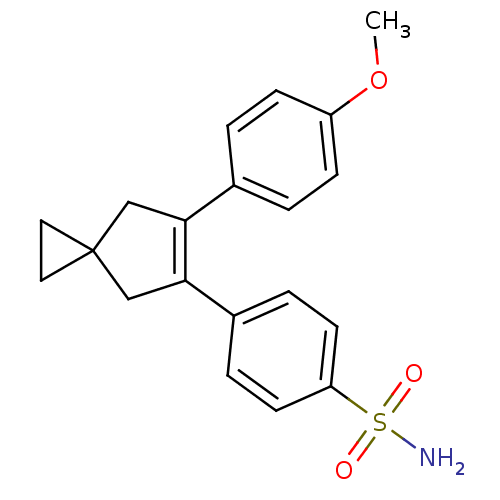
(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049014

(4-[6-(4-Chloro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C19H18ClNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049033
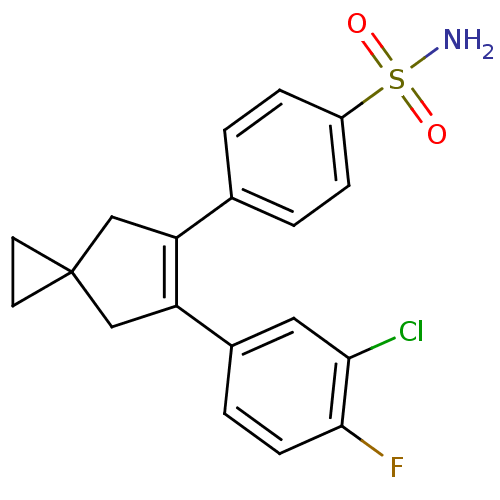
(4-[6-(3-Chloro-4-fluoro-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17ClFNO2S/c20-17-9-13(3-6-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049030
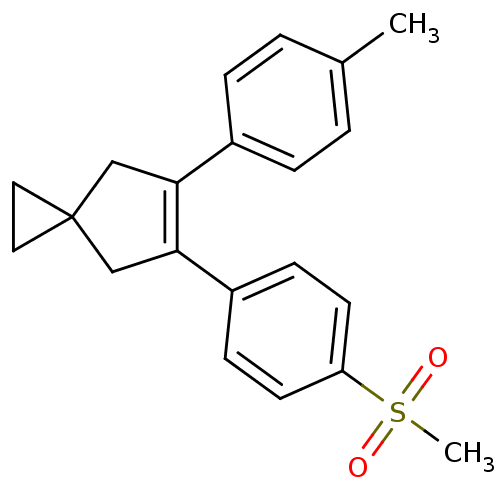
(5-(4-Methanesulfonyl-phenyl)-6-p-tolyl-spiro[2.4]h...)Show SMILES Cc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:8| Show InChI InChI=1S/C21H22O2S/c1-15-3-5-16(6-4-15)19-13-21(11-12-21)14-20(19)17-7-9-18(10-8-17)24(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049037
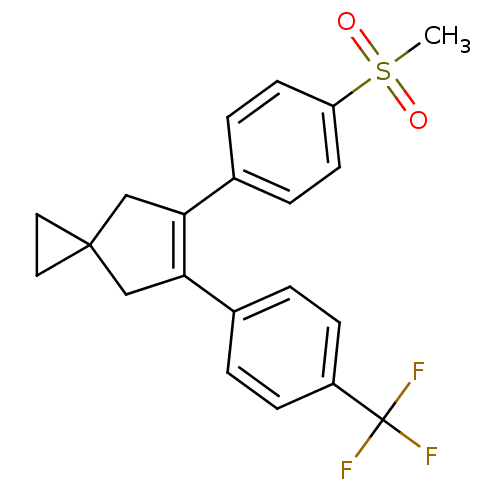
(5-(4-Methanesulfonyl-phenyl)-6-(4-trifluoromethyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C21H19F3O2S/c1-27(25,26)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(7-3-14)21(22,23)24/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049029

(4-[6-(3-Chloro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20ClNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049023
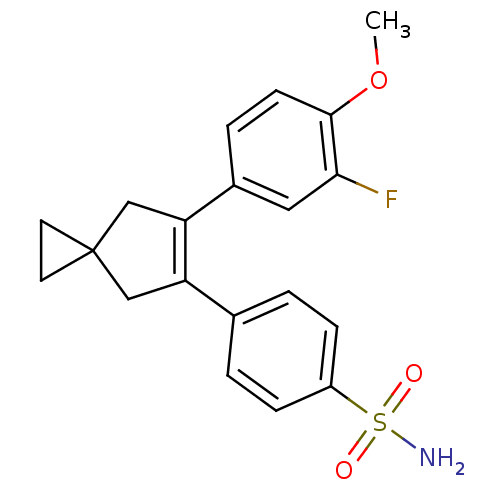
(4-[6-(3-Fluoro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1F)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20FNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049040
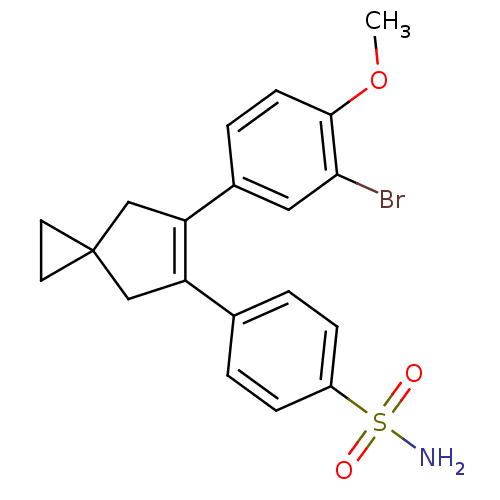
(4-[6-(3-Bromo-4-methoxy-phenyl)-spiro[2.4]hept-5-e...)Show SMILES COc1ccc(cc1Br)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20BrNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049028
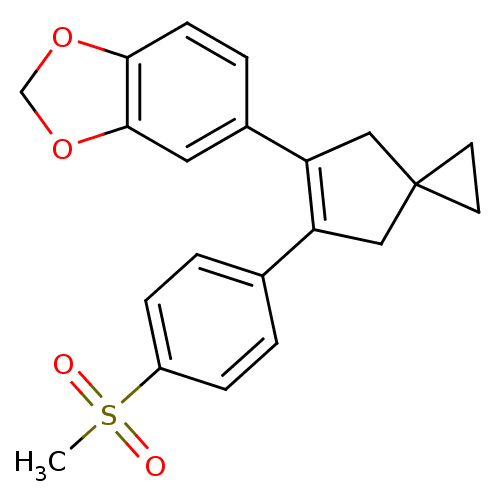
(5-[6-(4-Methanesulfonyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C21H20O4S/c1-26(22,23)16-5-2-14(3-6-16)17-11-21(8-9-21)12-18(17)15-4-7-19-20(10-15)25-13-24-19/h2-7,10H,8-9,11-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049026

(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=CC2(CC2)C=C1c1ccc(F)cc1 |c:17,t:11| Show InChI InChI=1S/C20H17FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9,12-13H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029614
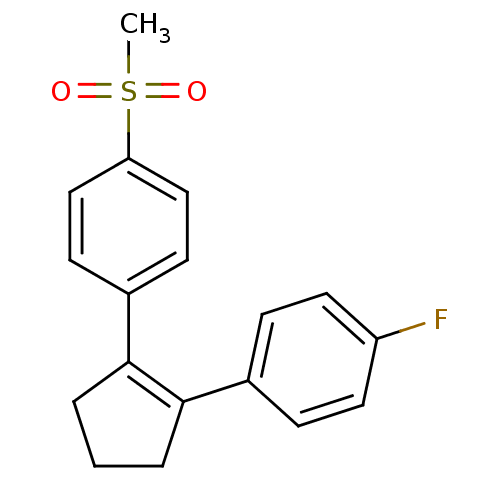
((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C18H17FO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049010

(4-[6-(3,4-Difluoro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C19H17F2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049024

(4-[6-(4-Fluoro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C19H18FNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049018

(5-(3,4-Dichloro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C20H18Cl2O2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-18(21)19(22)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049013
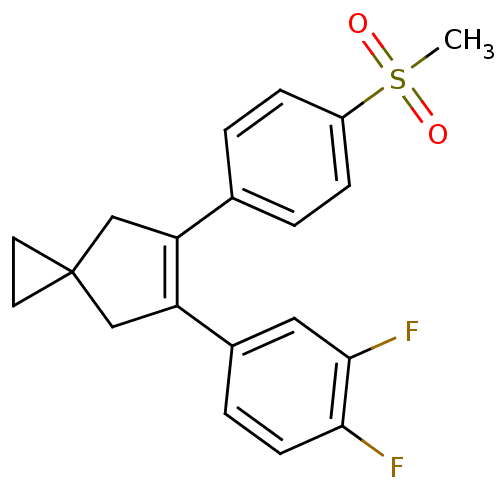
(5-(3,4-Difluoro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C20H18F2O2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-18(21)19(22)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049022

(6-(4-Fluoro-phenyl)-7-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CCC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C21H21FO2S/c1-25(23,24)18-9-5-16(6-10-18)20-14-21(11-2-12-21)13-19(20)15-3-7-17(22)8-4-15/h3-10H,2,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049027
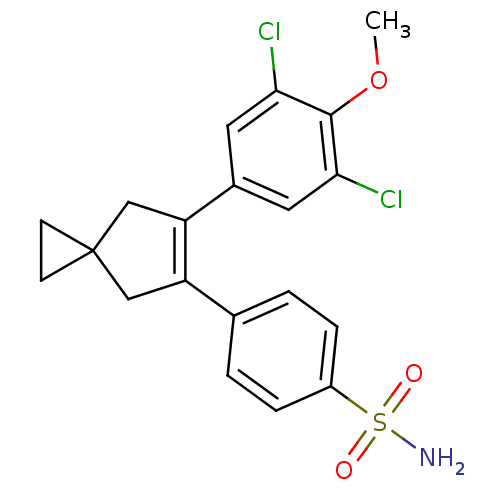
(4-[6-(3,5-Dichloro-4-methoxy-phenyl)-spiro[2.4]hep...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:11| Show InChI InChI=1S/C20H19Cl2NO3S/c1-26-19-17(21)8-13(9-18(19)22)16-11-20(6-7-20)10-15(16)12-2-4-14(5-3-12)27(23,24)25/h2-5,8-9H,6-7,10-11H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049034
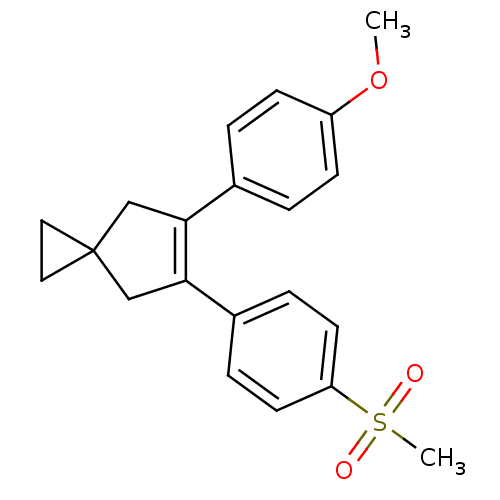
(5-(4-Methanesulfonyl-phenyl)-6-(4-methoxy-phenyl)-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C21H22O3S/c1-24-17-7-3-15(4-8-17)19-13-21(11-12-21)14-20(19)16-5-9-18(10-6-16)25(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049025

(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049031
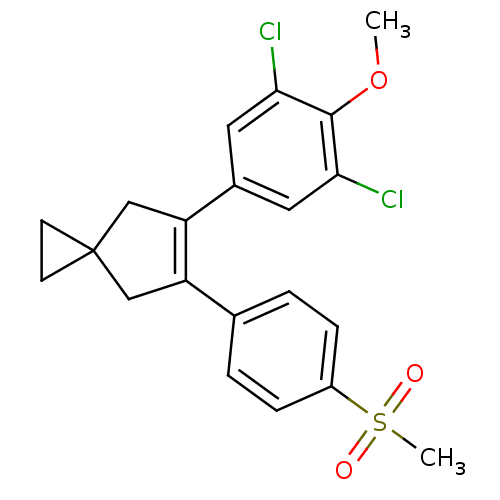
(5-(3,5-Dichloro-4-methoxy-phenyl)-6-(4-methanesulf...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C21H20Cl2O3S/c1-26-20-18(22)9-14(10-19(20)23)17-12-21(7-8-21)11-16(17)13-3-5-15(6-4-13)27(2,24)25/h3-6,9-10H,7-8,11-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049021

(5-(3-Chloro-4-fluoro-phenyl)-6-(4-methanesulfonyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C20H18ClFO2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-19(22)18(21)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049041
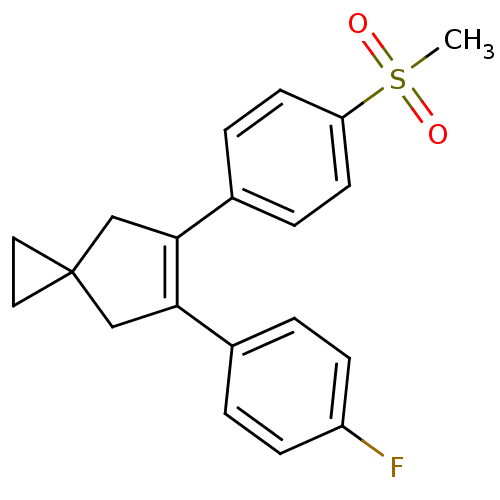
(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C20H19FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049035
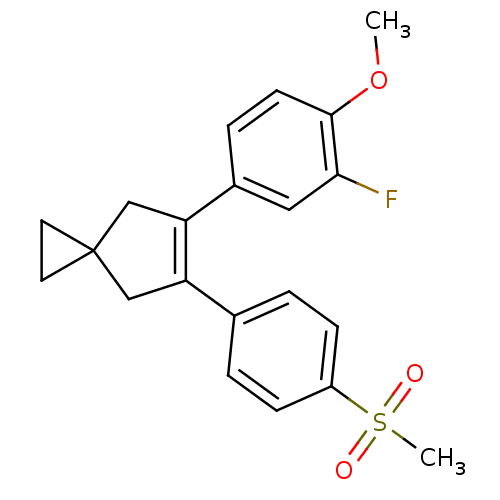
(5-(3-Fluoro-4-methoxy-phenyl)-6-(4-methanesulfonyl...)Show SMILES COc1ccc(cc1F)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C21H21FO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049032
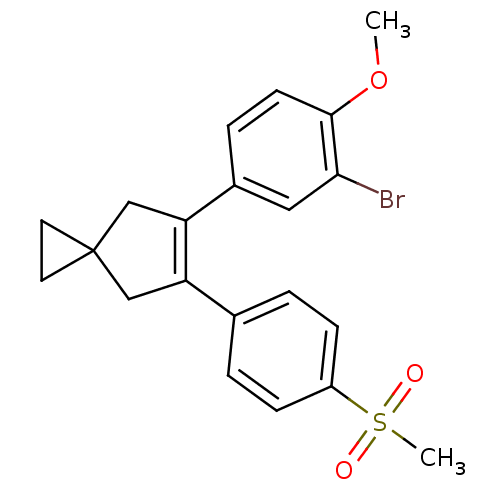
(5-(3-Bromo-4-methoxy-phenyl)-6-(4-methanesulfonyl-...)Show SMILES COc1ccc(cc1Br)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C21H21BrO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049019
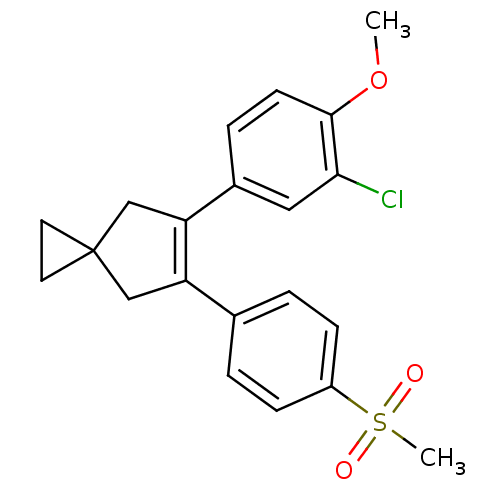
(5-(3-Chloro-4-methoxy-phenyl)-6-(4-methanesulfonyl...)Show SMILES COc1ccc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C21H21ClO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049017
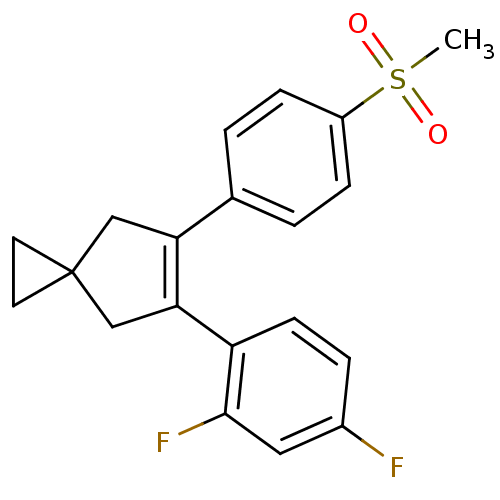
(5-(2,4-Difluoro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1F |t:11| Show InChI InChI=1S/C20H18F2O2S/c1-25(23,24)15-5-2-13(3-6-15)17-11-20(8-9-20)12-18(17)16-7-4-14(21)10-19(16)22/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049036
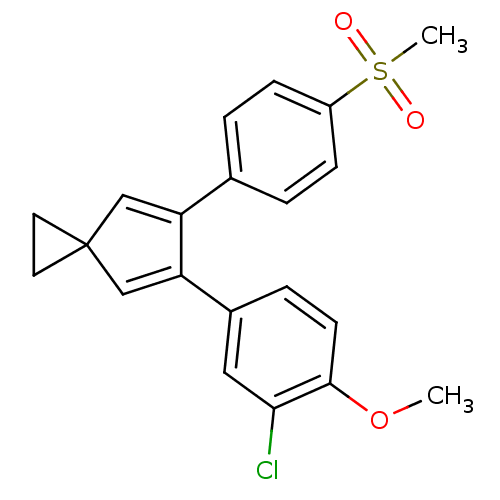
(5-(3-Chloro-4-methoxy-phenyl)-6-(4-methanesulfonyl...)Show SMILES COc1ccc(cc1Cl)C1=CC2(CC2)C=C1c1ccc(cc1)S(C)(=O)=O |c:16,t:10| Show InChI InChI=1S/C21H19ClO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11-13H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049020
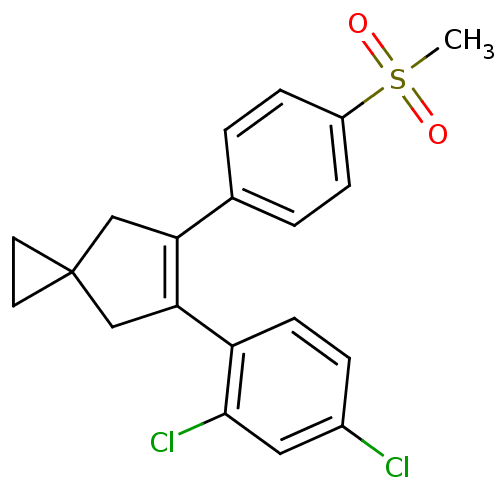
(5-(2,4-Dichloro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1Cl |t:11| Show InChI InChI=1S/C20H18Cl2O2S/c1-25(23,24)15-5-2-13(3-6-15)17-11-20(8-9-20)12-18(17)16-7-4-14(21)10-19(16)22/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049039
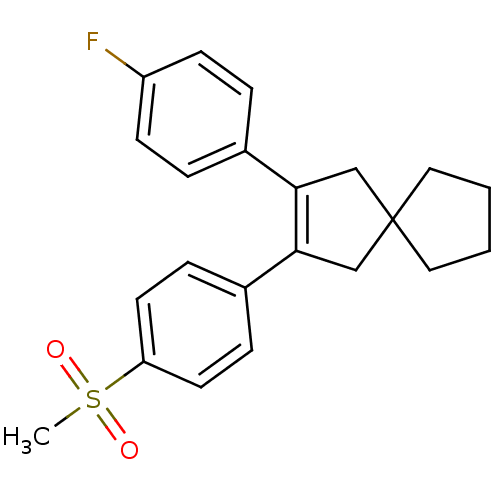
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CCCC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C22H23FO2S/c1-26(24,25)19-10-6-17(7-11-19)21-15-22(12-2-3-13-22)14-20(21)16-4-8-18(23)9-5-16/h4-11H,2-3,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049016
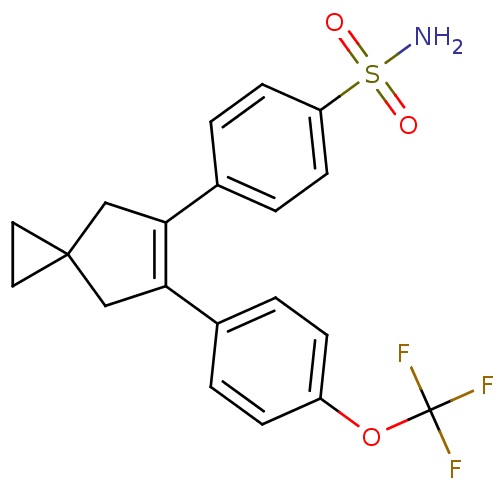
(4-[6-(4-Trifluoromethoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(OC(F)(F)F)cc1 |t:11| Show InChI InChI=1S/C20H18F3NO3S/c21-20(22,23)27-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)28(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
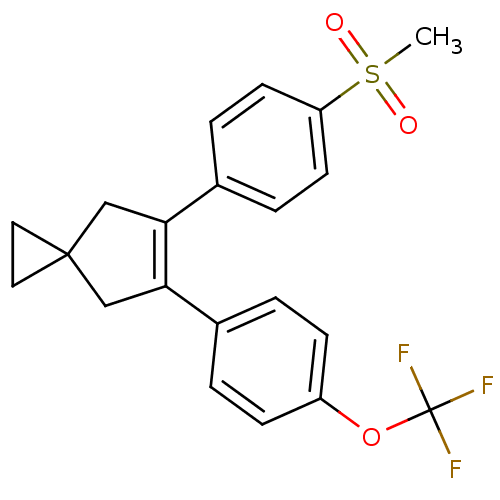
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049015
(5-(4-Methanesulfonyl-phenyl)-6-(4-trifluoromethoxy...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(OC(F)(F)F)cc1 |t:11| Show InChI InChI=1S/C21H19F3O3S/c1-28(25,26)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(7-3-14)27-21(22,23)24/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049014

(4-[6-(4-Chloro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C19H18ClNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049033

(4-[6-(3-Chloro-4-fluoro-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17ClFNO2S/c20-17-9-13(3-6-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049011

(4-[6-(3,4-Dichloro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17Cl2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049040

(4-[6-(3-Bromo-4-methoxy-phenyl)-spiro[2.4]hept-5-e...)Show SMILES COc1ccc(cc1Br)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20BrNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049023

(4-[6-(3-Fluoro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1F)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20FNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049042

(4-[6-(4-Trifluoromethyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C20H18F3NO2S/c21-20(22,23)15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)27(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049034

(5-(4-Methanesulfonyl-phenyl)-6-(4-methoxy-phenyl)-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C21H22O3S/c1-24-17-7-3-15(4-8-17)19-13-21(11-12-21)14-20(19)16-5-9-18(10-6-16)25(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049017

(5-(2,4-Difluoro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1F |t:11| Show InChI InChI=1S/C20H18F2O2S/c1-25(23,24)15-5-2-13(3-6-15)17-11-20(8-9-20)12-18(17)16-7-4-14(21)10-19(16)22/h2-7,10H,8-9,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049010

(4-[6-(3,4-Difluoro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C19H17F2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049026

(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=CC2(CC2)C=C1c1ccc(F)cc1 |c:17,t:11| Show InChI InChI=1S/C20H17FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9,12-13H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049028

(5-[6-(4-Methanesulfonyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C21H20O4S/c1-26(22,23)16-5-2-14(3-6-16)17-11-21(8-9-21)12-18(17)15-4-7-19-20(10-15)25-13-24-19/h2-7,10H,8-9,11-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049016

(4-[6-(4-Trifluoromethoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(OC(F)(F)F)cc1 |t:11| Show InChI InChI=1S/C20H18F3NO3S/c21-20(22,23)27-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)28(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049029

(4-[6-(3-Chloro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20ClNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049038

(5-(4-Chloro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C20H19ClO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049036

(5-(3-Chloro-4-methoxy-phenyl)-6-(4-methanesulfonyl...)Show SMILES COc1ccc(cc1Cl)C1=CC2(CC2)C=C1c1ccc(cc1)S(C)(=O)=O |c:16,t:10| Show InChI InChI=1S/C21H19ClO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11-13H,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049030

(5-(4-Methanesulfonyl-phenyl)-6-p-tolyl-spiro[2.4]h...)Show SMILES Cc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:8| Show InChI InChI=1S/C21H22O2S/c1-15-3-5-16(6-4-15)19-13-21(11-12-21)14-20(19)17-7-9-18(10-8-17)24(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data