

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015009 (CHEMBL3262367 | US9259422, 7a, R = 3,5-diMePh- BU1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015000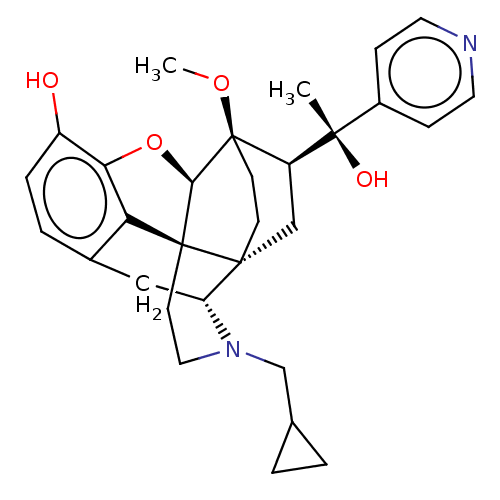 (CHEMBL3262377) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015011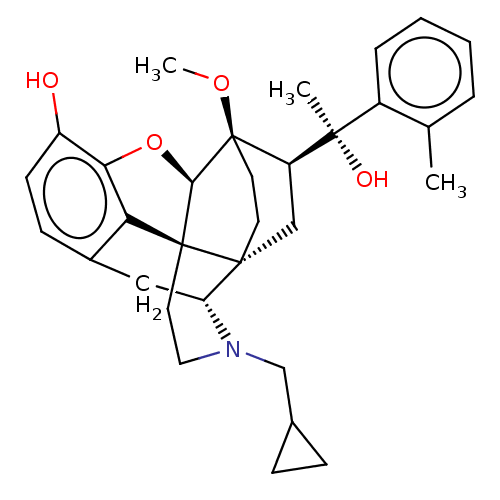 (CHEMBL3262363 | US9259422, 7a, 2-MePh- BU10101 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015011 (CHEMBL3262363 | US9259422, 7a, 2-MePh- BU10101 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015009 (CHEMBL3262367 | US9259422, 7a, R = 3,5-diMePh- BU1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015010 (CHEMBL3262091) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015000 (CHEMBL3262377) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015007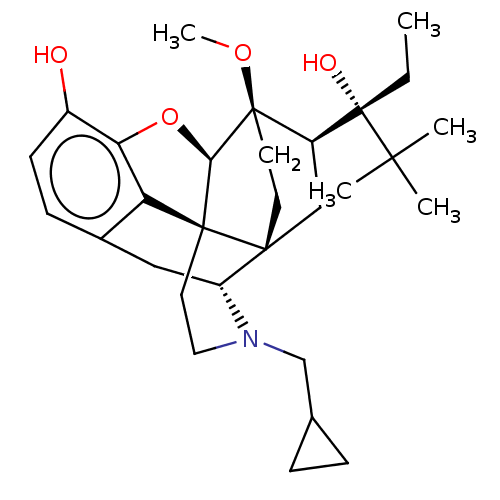 (CHEMBL3262088) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015004 (CHEMBL3262372 | US9259422, 7a, R = 2-thienyl- BU08...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015008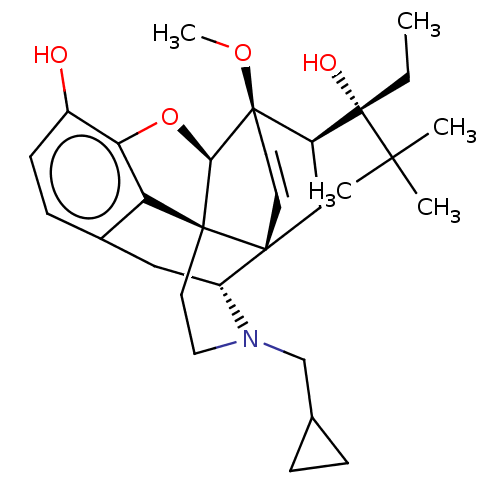 (CHEMBL3262362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015010 (CHEMBL3262091) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50015006 (CHEMBL3262360) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mu opioid receptor in Hartley guinea pig brain membranes after 60 mins by beta-plate liquid scintillation counting an... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015001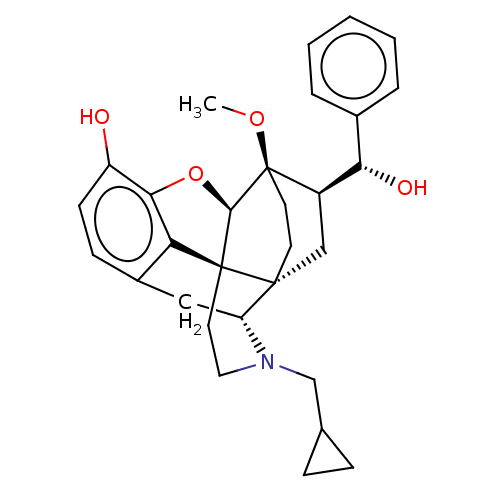 (CHEMBL3262092) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015007 (CHEMBL3262088) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015001 (CHEMBL3262092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50014998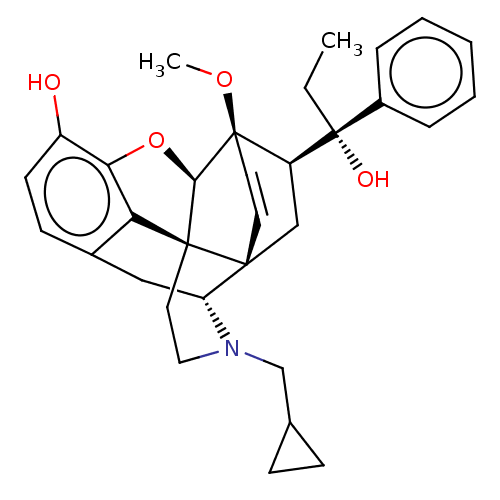 (CHEMBL3262361) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015000 (CHEMBL3262377) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015004 (CHEMBL3262372 | US9259422, 7a, R = 2-thienyl- BU08...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015010 (CHEMBL3262091) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015008 (CHEMBL3262362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50014998 (CHEMBL3262361) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50014995 (CHEMBL3262090 | US9259422, 13a, R = Ph-BU126 | US9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015001 (CHEMBL3262092) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50015006 (CHEMBL3262360) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from kappa opioid receptor in Hartley guinea pig brain membranes after 60 mins by beta-plate liquid scintillation counti... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50014995 (CHEMBL3262090 | US9259422, 13a, R = Ph-BU126 | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015004 (CHEMBL3262372 | US9259422, 7a, R = 2-thienyl- BU08...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50014998 (CHEMBL3262361) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50015005 (CHEMBL3262093 | US9259422, 13b, R = Ph-BU125 | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015005 (CHEMBL3262093 | US9259422, 13b, R = Ph-BU125 | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015005 (CHEMBL3262093 | US9259422, 13b, R = Ph-BU125 | US9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015007 (CHEMBL3262088) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015008 (CHEMBL3262362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analysis | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50014995 (CHEMBL3262090 | US9259422, 13a, R = Ph-BU126 | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting anal... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from human delta opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting analys... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from recombinant nociceptin opioid receptor (unknown origin) expressed in HEK cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50015004 (CHEMBL3262372 | US9259422, 7a, R = 2-thienyl- BU08...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from recombinant nociceptin opioid receptor (unknown origin) expressed in HEK cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from human nociceptin opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting a... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50015007 (CHEMBL3262088) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from human nociceptin opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting a... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50015008 (CHEMBL3262362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from human nociceptin opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting a... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50014998 (CHEMBL3262361) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-N/OFQ from human nociceptin opioid receptor transfected in CHO cells after 60 mins by beta-plate liquid scintillation counting a... | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |