 Found 83 hits Enz. Inhib. hit(s) with all data for entry = 50044445
Found 83 hits Enz. Inhib. hit(s) with all data for entry = 50044445 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017300
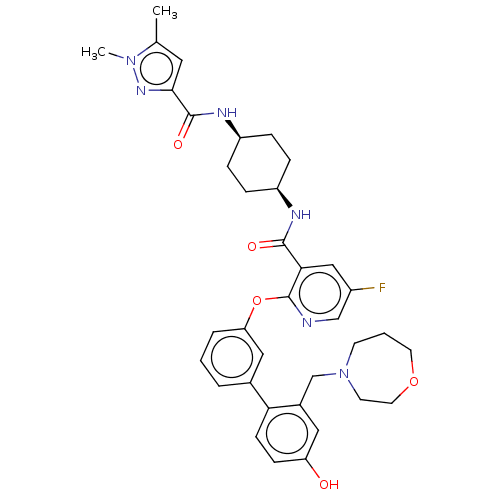
(CHEMBL3287987)Show SMILES Cc1cc(nn1C)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCCOCC1 |r,wU:13.17,10.10,(38.69,-2.82,;38.26,-4.29,;36.82,-4.82,;36.87,-6.34,;38.34,-6.79,;39.21,-5.51,;40.75,-5.46,;35.65,-7.29,;35.65,-8.83,;34.31,-6.52,;32.98,-7.29,;32.98,-8.83,;31.65,-9.6,;30.31,-8.82,;30.31,-7.29,;31.64,-6.52,;28.98,-9.6,;27.64,-8.83,;27.64,-7.29,;26.31,-9.61,;24.98,-8.84,;23.65,-9.61,;22.31,-8.84,;23.65,-11.16,;24.98,-11.93,;26.32,-11.16,;27.65,-11.92,;27.65,-13.46,;26.32,-14.23,;26.32,-15.77,;27.66,-16.54,;28.99,-15.76,;28.99,-14.22,;30.33,-16.52,;30.33,-18.06,;31.67,-18.82,;33,-18.05,;34.34,-18.81,;32.99,-16.5,;31.65,-15.74,;31.64,-14.2,;32.97,-13.42,;32.84,-11.9,;33.96,-10.84,;35.48,-11.04,;36.26,-12.37,;35.72,-13.81,;34.25,-14.28,)| Show InChI InChI=1S/C36H41FN6O5/c1-23-17-33(41-42(23)2)35(46)40-28-9-7-27(8-10-28)39-34(45)32-20-26(37)21-38-36(32)48-30-6-3-5-24(19-30)31-12-11-29(44)18-25(31)22-43-13-4-15-47-16-14-43/h3,5-6,11-12,17-21,27-28,44H,4,7-10,13-16,22H2,1-2H3,(H,39,45)(H,40,46)/t27-,28+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415001
(CHEMBL570015 | GSK-256066 | GSK-256066 (3))Show SMILES COc1cccc(Nc2c(cnc3c(C)cc(cc23)S(=O)(=O)c2cccc(c2)C(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C27H26N4O5S/c1-16-11-21(37(34,35)20-10-5-7-17(12-20)27(33)31(2)3)14-22-24(16)29-15-23(26(28)32)25(22)30-18-8-6-9-19(13-18)36-4/h5-15H,1-4H3,(H2,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017304
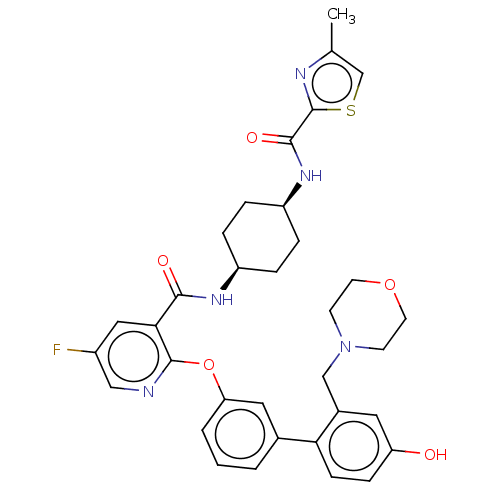
(CHEMBL3287991)Show SMILES Cc1csc(n1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:9.9,12.16,(40.08,-42.78,;38.55,-42.67,;37.73,-41.37,;36.21,-41.6,;36.13,-43.26,;37.56,-43.85,;34.83,-44.08,;34.83,-45.62,;33.5,-43.3,;32.16,-44.08,;32.15,-45.62,;30.83,-46.38,;29.49,-45.61,;29.48,-44.08,;30.82,-43.3,;28.16,-46.38,;26.82,-45.62,;26.82,-44.08,;25.49,-46.39,;24.16,-45.63,;22.82,-46.4,;21.49,-45.63,;22.82,-47.94,;24.16,-48.72,;25.5,-47.94,;26.83,-48.71,;26.83,-50.25,;25.5,-51.02,;25.5,-52.56,;26.84,-53.33,;28.17,-52.55,;28.16,-51.01,;29.51,-53.31,;29.51,-54.85,;30.85,-55.61,;32.18,-54.84,;33.51,-55.6,;32.16,-53.29,;30.83,-52.53,;30.82,-50.99,;32.14,-50.21,;33.48,-50.97,;34.81,-50.2,;34.8,-48.65,;33.45,-47.89,;32.12,-48.68,)| Show InChI InChI=1S/C34H36FN5O5S/c1-21-20-46-34(37-21)32(43)39-26-7-5-25(6-8-26)38-31(42)30-17-24(35)18-36-33(30)45-28-4-2-3-22(16-28)29-10-9-27(41)15-23(29)19-40-11-13-44-14-12-40/h2-4,9-10,15-18,20,25-26,41H,5-8,11-14,19H2,1H3,(H,38,42)(H,39,43)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017296

(CHEMBL3287739)Show SMILES Cc1cc(nn1C)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:13.17,10.10,(19.48,-15.99,;19.06,-17.47,;17.61,-18,;17.66,-19.52,;19.13,-19.96,;20,-18.69,;21.54,-18.64,;16.44,-20.46,;16.44,-22,;15.1,-19.69,;13.77,-20.46,;13.77,-22,;12.44,-22.77,;11.1,-22,;11.1,-20.46,;12.43,-19.69,;9.77,-22.77,;8.43,-22.01,;8.43,-20.47,;7.1,-22.78,;5.77,-22.02,;4.44,-22.79,;3.1,-22.02,;4.44,-24.33,;5.77,-25.1,;7.11,-24.33,;8.44,-25.1,;8.45,-26.64,;7.11,-27.41,;7.11,-28.95,;8.45,-29.72,;9.78,-28.94,;9.78,-27.4,;11.12,-29.69,;11.12,-31.24,;12.46,-32,;13.79,-31.22,;15.13,-31.98,;13.78,-29.68,;12.44,-28.92,;12.43,-27.38,;13.76,-26.6,;15.09,-27.36,;16.42,-26.58,;16.41,-25.04,;15.06,-24.28,;13.74,-25.06,)| Show InChI InChI=1S/C35H39FN6O5/c1-22-16-32(40-41(22)2)34(45)39-27-8-6-26(7-9-27)38-33(44)31-19-25(36)20-37-35(31)47-29-5-3-4-23(18-29)30-11-10-28(43)17-24(30)21-42-12-14-46-15-13-42/h3-5,10-11,16-20,26-27,43H,6-9,12-15,21H2,1-2H3,(H,38,44)(H,39,45)/t26-,27+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017308
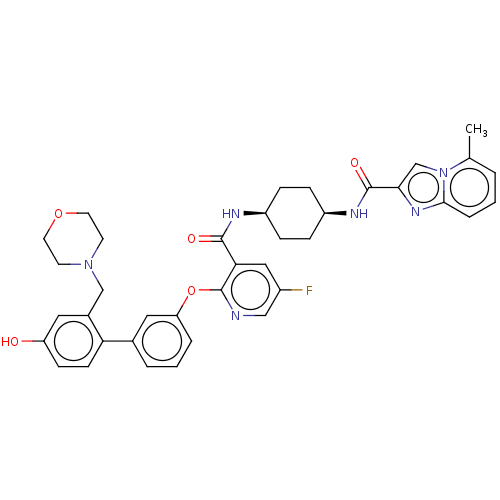
(CHEMBL3287995)Show SMILES Cc1cccc2nc(cn12)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:13.14,16.21,(37.17,-18.1,;38.11,-19.31,;39.63,-19.1,;40.58,-20.32,;39.99,-21.74,;38.48,-21.94,;37.62,-23.22,;36.14,-22.77,;36.09,-21.25,;37.54,-20.73,;34.92,-23.72,;34.92,-25.26,;33.59,-22.95,;32.24,-23.72,;32.24,-25.26,;30.91,-26.03,;29.57,-25.26,;29.57,-23.72,;30.91,-22.95,;28.24,-26.03,;26.91,-25.27,;26.9,-23.73,;25.57,-26.04,;24.24,-25.28,;22.9,-26.05,;21.57,-25.28,;22.9,-27.59,;24.24,-28.37,;25.58,-27.59,;26.91,-28.36,;26.92,-29.91,;25.58,-30.67,;25.58,-32.21,;26.92,-32.99,;28.26,-32.2,;28.25,-30.66,;29.59,-32.96,;29.6,-34.51,;30.93,-35.27,;32.27,-34.49,;33.6,-35.25,;32.25,-32.94,;30.92,-32.18,;30.9,-30.64,;32.23,-29.86,;33.57,-30.63,;34.89,-29.86,;34.89,-28.32,;33.55,-27.56,;32.22,-28.33,)| Show InChI InChI=1S/C38H39FN6O5/c1-24-4-2-7-35-43-34(23-45(24)35)37(48)42-29-10-8-28(9-11-29)41-36(47)33-20-27(39)21-40-38(33)50-31-6-3-5-25(19-31)32-13-12-30(46)18-26(32)22-44-14-16-49-17-15-44/h2-7,12-13,18-21,23,28-29,46H,8-11,14-17,22H2,1H3,(H,41,47)(H,42,48)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50017294
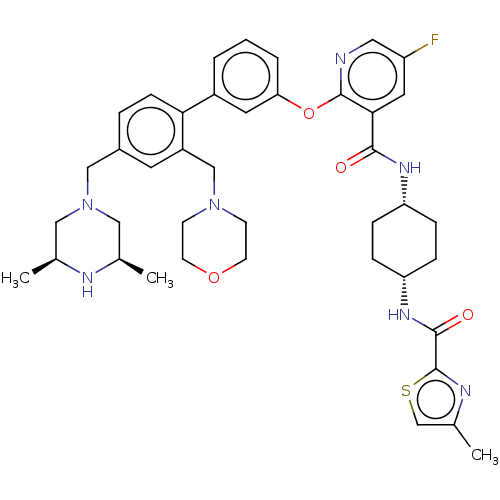
(CHEMBL3288029)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,1.0,51.57,(20.55,-53.47,;19.22,-52.7,;17.9,-53.47,;16.57,-52.7,;15.24,-53.48,;13.91,-52.72,;12.58,-53.49,;11.24,-52.73,;11.24,-51.19,;12.56,-50.4,;12.55,-48.86,;13.88,-48.09,;15.21,-48.85,;16.54,-48.08,;16.53,-46.54,;15.2,-45.78,;13.86,-46.55,;13.9,-51.17,;9.9,-50.42,;8.56,-51.21,;7.23,-50.43,;7.22,-48.89,;8.56,-48.13,;8.56,-46.58,;7.22,-45.81,;5.88,-46.59,;4.54,-45.81,;4.54,-44.27,;3.21,-43.5,;5.88,-43.5,;7.22,-44.26,;8.55,-43.49,;8.54,-41.94,;9.88,-44.25,;11.22,-43.48,;12.56,-44.25,;13.89,-43.48,;13.89,-41.94,;12.55,-41.16,;11.21,-41.94,;15.23,-41.16,;16.57,-41.94,;16.57,-43.48,;17.78,-40.99,;19.26,-41.44,;20.12,-40.16,;21.66,-40.11,;19.18,-38.94,;17.73,-39.47,;9.89,-48.88,;16.55,-51.17,;17.88,-50.4,;17.88,-48.87,;19.22,-51.17,)| Show InChI InChI=1S/C41H50FN7O4S/c1-26-21-49(22-27(2)44-26)23-29-7-12-36(31(17-29)24-48-13-15-52-16-14-48)30-5-4-6-35(18-30)53-40-37(19-32(42)20-43-40)38(50)46-33-8-10-34(11-9-33)47-39(51)41-45-28(3)25-54-41/h4-7,12,17-20,25-27,33-34,44H,8-11,13-16,21-24H2,1-3H3,(H,46,50)(H,47,51)/t26-,27+,33-,34+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50017295
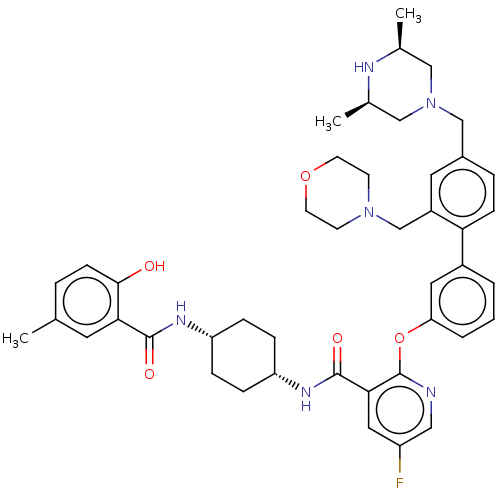
(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50017294

(CHEMBL3288029)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,1.0,51.57,(20.55,-53.47,;19.22,-52.7,;17.9,-53.47,;16.57,-52.7,;15.24,-53.48,;13.91,-52.72,;12.58,-53.49,;11.24,-52.73,;11.24,-51.19,;12.56,-50.4,;12.55,-48.86,;13.88,-48.09,;15.21,-48.85,;16.54,-48.08,;16.53,-46.54,;15.2,-45.78,;13.86,-46.55,;13.9,-51.17,;9.9,-50.42,;8.56,-51.21,;7.23,-50.43,;7.22,-48.89,;8.56,-48.13,;8.56,-46.58,;7.22,-45.81,;5.88,-46.59,;4.54,-45.81,;4.54,-44.27,;3.21,-43.5,;5.88,-43.5,;7.22,-44.26,;8.55,-43.49,;8.54,-41.94,;9.88,-44.25,;11.22,-43.48,;12.56,-44.25,;13.89,-43.48,;13.89,-41.94,;12.55,-41.16,;11.21,-41.94,;15.23,-41.16,;16.57,-41.94,;16.57,-43.48,;17.78,-40.99,;19.26,-41.44,;20.12,-40.16,;21.66,-40.11,;19.18,-38.94,;17.73,-39.47,;9.89,-48.88,;16.55,-51.17,;17.88,-50.4,;17.88,-48.87,;19.22,-51.17,)| Show InChI InChI=1S/C41H50FN7O4S/c1-26-21-49(22-27(2)44-26)23-29-7-12-36(31(17-29)24-48-13-15-52-16-14-48)30-5-4-6-35(18-30)53-40-37(19-32(42)20-43-40)38(50)46-33-8-10-34(11-9-33)47-39(51)41-45-28(3)25-54-41/h4-7,12,17-20,25-27,33-34,44H,8-11,13-16,21-24H2,1-3H3,(H,46,50)(H,47,51)/t26-,27+,33-,34+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat PDE4B |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017303
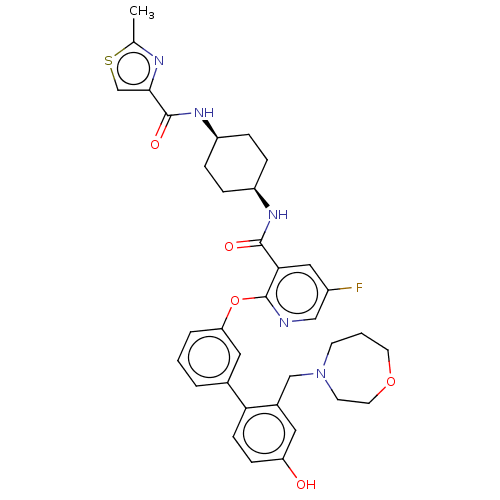
(CHEMBL3287990)Show SMILES Cc1nc(cs1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCCOCC1 |r,wU:12.16,9.9,(25.66,-22.69,;24.12,-22.74,;23.26,-24.01,;21.78,-23.57,;21.73,-22.05,;23.18,-21.52,;20.56,-24.51,;20.56,-26.05,;19.23,-23.74,;17.89,-24.51,;17.89,-26.05,;16.56,-26.82,;15.22,-26.05,;15.22,-24.52,;16.56,-23.74,;13.89,-26.82,;12.56,-26.06,;12.55,-24.52,;11.22,-26.83,;9.89,-26.07,;8.56,-26.84,;7.23,-26.07,;8.56,-28.38,;9.89,-29.15,;11.23,-28.38,;12.56,-29.15,;12.57,-30.69,;11.23,-31.46,;11.23,-33,;12.57,-33.77,;13.91,-32.99,;13.9,-31.45,;15.24,-33.75,;15.25,-35.29,;16.58,-36.05,;17.91,-35.27,;19.25,-36.04,;17.9,-33.73,;16.56,-32.97,;16.55,-31.43,;17.88,-30.65,;17.75,-29.12,;18.87,-28.06,;20.4,-28.27,;21.17,-29.6,;20.63,-31.04,;19.16,-31.51,)| Show InChI InChI=1S/C35H38FN5O5S/c1-22-38-32(21-47-22)34(44)40-27-8-6-26(7-9-27)39-33(43)31-18-25(36)19-37-35(31)46-29-5-2-4-23(17-29)30-11-10-28(42)16-24(30)20-41-12-3-14-45-15-13-41/h2,4-5,10-11,16-19,21,26-27,42H,3,6-9,12-15,20H2,1H3,(H,39,43)(H,40,44)/t26-,27+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017307
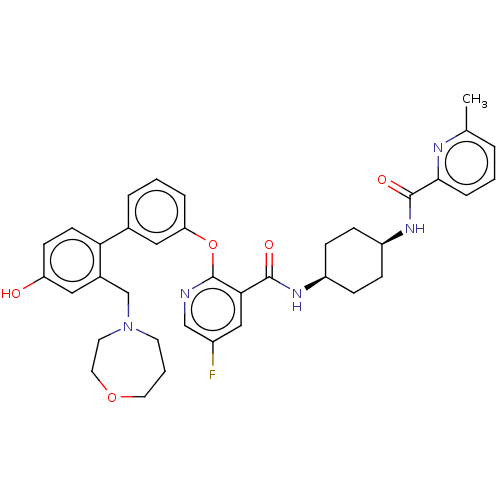
(CHEMBL3287994)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCCOCC1 |r,wU:10.10,13.17,(21.13,-24.41,;19.8,-23.64,;19.81,-22.1,;18.47,-21.33,;17.14,-22.1,;17.15,-23.63,;18.47,-24.4,;15.82,-24.4,;15.82,-25.94,;14.49,-23.62,;13.14,-24.4,;13.14,-25.94,;11.81,-26.71,;10.47,-25.93,;10.47,-24.4,;11.81,-23.62,;9.14,-26.71,;7.8,-25.94,;7.8,-24.4,;6.47,-26.72,;5.13,-25.96,;3.8,-26.72,;2.46,-25.96,;3.8,-28.27,;5.14,-29.05,;6.48,-28.27,;7.81,-29.04,;7.81,-30.58,;6.48,-31.35,;6.48,-32.89,;7.82,-33.67,;9.16,-32.88,;9.15,-31.34,;10.49,-33.64,;10.5,-35.19,;11.83,-35.95,;13.17,-35.17,;14.51,-35.93,;13.15,-33.62,;11.81,-32.86,;11.8,-31.32,;13.13,-30.54,;13.01,-29.01,;14.13,-27.96,;15.65,-28.17,;16.43,-29.49,;15.88,-30.93,;14.41,-31.4,)| Show InChI InChI=1S/C37H40FN5O5/c1-24-5-2-8-34(40-24)36(46)42-29-11-9-28(10-12-29)41-35(45)33-21-27(38)22-39-37(33)48-31-7-3-6-25(20-31)32-14-13-30(44)19-26(32)23-43-15-4-17-47-18-16-43/h2-3,5-8,13-14,19-22,28-29,44H,4,9-12,15-18,23H2,1H3,(H,41,45)(H,42,46)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017313
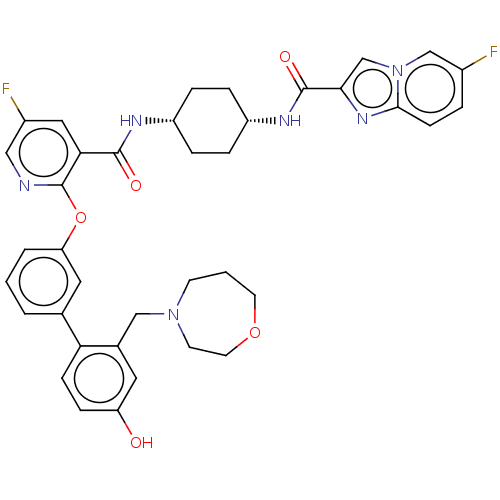
(CHEMBL3287999)Show SMILES Oc1ccc(c(CN2CCCOCC2)c1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cn3cc(F)ccc3n2)c1 |r,wU:34.40,31.33,(13.4,-36.59,;12.06,-35.83,;10.73,-36.61,;9.39,-35.85,;9.39,-34.3,;10.71,-33.52,;10.7,-31.98,;12.02,-31.2,;11.9,-29.67,;13.02,-28.62,;14.54,-28.83,;15.32,-30.15,;14.77,-31.59,;13.3,-32.06,;12.05,-34.28,;8.05,-33.54,;6.71,-34.32,;5.38,-33.55,;5.38,-32.01,;6.71,-31.24,;6.71,-29.7,;5.37,-28.93,;4.04,-29.71,;2.69,-28.93,;2.7,-27.38,;1.36,-26.62,;4.03,-26.62,;5.37,-27.38,;6.7,-26.61,;6.69,-25.06,;8.04,-27.37,;9.37,-26.6,;10.71,-27.37,;12.03,-26.6,;12.04,-25.06,;10.7,-24.29,;9.36,-25.06,;13.38,-24.29,;14.71,-25.06,;14.71,-26.6,;15.93,-24.11,;15.88,-22.59,;17.33,-22.07,;17.91,-20.65,;19.42,-20.44,;20,-19.02,;20.37,-21.66,;19.79,-23.08,;18.27,-23.28,;17.41,-24.56,;8.04,-32,)| Show InChI InChI=1S/C38H38F2N6O5/c39-26-5-12-35-44-34(23-46(35)22-26)37(49)43-29-8-6-28(7-9-29)42-36(48)33-19-27(40)20-41-38(33)51-31-4-1-3-24(18-31)32-11-10-30(47)17-25(32)21-45-13-2-15-50-16-14-45/h1,3-5,10-12,17-20,22-23,28-29,47H,2,6-9,13-16,21H2,(H,42,48)(H,43,49)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017338
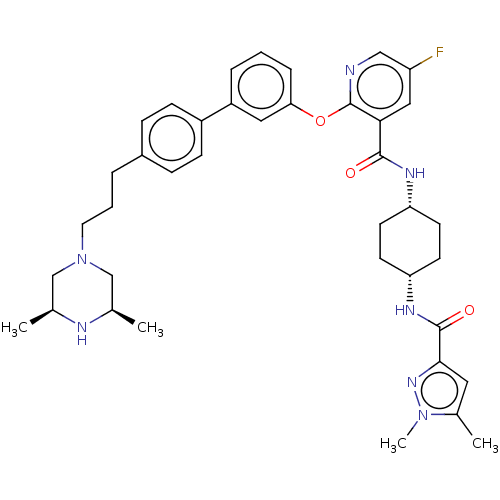
(CHEMBL3288027)Show SMILES C[C@H]1CN(CCCc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:32.37,29.30,47.52,1.0,(24.33,-19.69,;23,-18.93,;21.68,-19.7,;20.35,-18.93,;19.02,-19.7,;17.69,-18.94,;16.36,-19.72,;15.03,-18.96,;13.7,-19.73,;12.36,-18.97,;12.35,-17.43,;13.68,-16.65,;15.02,-17.41,;11.02,-16.66,;9.68,-17.45,;8.34,-16.67,;8.34,-15.13,;9.68,-14.37,;9.67,-12.82,;8.34,-12.05,;7,-12.83,;5.66,-12.05,;5.66,-10.5,;4.32,-9.74,;7,-9.74,;8.33,-10.5,;9.67,-9.73,;9.66,-8.18,;11,-10.49,;12.34,-9.72,;13.68,-10.49,;15.01,-9.72,;15.01,-8.18,;13.67,-7.4,;12.33,-8.18,;16.35,-7.4,;17.68,-8.18,;17.68,-9.72,;18.9,-7.23,;18.85,-5.7,;20.3,-5.18,;20.73,-3.71,;21.25,-6.4,;22.79,-6.35,;20.38,-7.67,;11.01,-15.12,;20.33,-17.4,;21.66,-16.63,;21.66,-15.09,;23,-17.39,)| Show InChI InChI=1S/C39H48FN7O3/c1-25-23-47(24-26(2)42-25)18-6-7-28-10-12-29(13-11-28)30-8-5-9-34(20-30)50-39-35(21-31(40)22-41-39)37(48)43-32-14-16-33(17-15-32)44-38(49)36-19-27(3)46(4)45-36/h5,8-13,19-22,25-26,32-33,42H,6-7,14-18,23-24H2,1-4H3,(H,43,48)(H,44,49)/t25-,26+,32-,33+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017295

(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017335

(CHEMBL3288024)Show SMILES C[C@H]1CN(Cc2cc(ccc2O)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:46.51,1.0,31.36,28.29,(17.3,-40.69,;17.31,-39.15,;16,-38.38,;16.01,-36.85,;14.65,-36.13,;13.33,-36.91,;11.99,-36.15,;10.67,-36.93,;10.67,-38.47,;12.01,-39.23,;13.34,-38.46,;14.68,-39.22,;9.33,-36.17,;8,-36.95,;6.66,-36.18,;6.66,-34.64,;7.99,-33.87,;7.99,-32.33,;6.65,-31.56,;5.32,-32.33,;3.97,-31.56,;3.98,-30.01,;2.64,-29.24,;5.31,-29.24,;6.65,-30,;7.98,-29.23,;7.97,-27.69,;9.32,-29.99,;10.65,-29.22,;11.99,-29.99,;13.32,-29.23,;13.32,-27.68,;11.98,-26.91,;10.65,-27.69,;14.66,-26.91,;16,-27.68,;16,-29.23,;17.22,-26.73,;17.17,-25.21,;18.62,-24.69,;19.05,-23.21,;19.56,-25.91,;21.1,-25.86,;18.7,-27.18,;9.32,-34.63,;17.33,-36.08,;18.66,-36.86,;20,-36.1,;18.65,-38.4,)| Show InChI InChI=1S/C37H44FN7O4/c1-22-19-45(20-23(2)40-22)21-27-15-26(8-13-34(27)46)25-6-5-7-31(16-25)49-37-32(17-28(38)18-39-37)35(47)41-29-9-11-30(12-10-29)42-36(48)33-14-24(3)44(4)43-33/h5-8,13-18,22-23,29-30,40,46H,9-12,19-21H2,1-4H3,(H,41,47)(H,42,48)/t22-,23+,29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017294

(CHEMBL3288029)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,1.0,51.57,(20.55,-53.47,;19.22,-52.7,;17.9,-53.47,;16.57,-52.7,;15.24,-53.48,;13.91,-52.72,;12.58,-53.49,;11.24,-52.73,;11.24,-51.19,;12.56,-50.4,;12.55,-48.86,;13.88,-48.09,;15.21,-48.85,;16.54,-48.08,;16.53,-46.54,;15.2,-45.78,;13.86,-46.55,;13.9,-51.17,;9.9,-50.42,;8.56,-51.21,;7.23,-50.43,;7.22,-48.89,;8.56,-48.13,;8.56,-46.58,;7.22,-45.81,;5.88,-46.59,;4.54,-45.81,;4.54,-44.27,;3.21,-43.5,;5.88,-43.5,;7.22,-44.26,;8.55,-43.49,;8.54,-41.94,;9.88,-44.25,;11.22,-43.48,;12.56,-44.25,;13.89,-43.48,;13.89,-41.94,;12.55,-41.16,;11.21,-41.94,;15.23,-41.16,;16.57,-41.94,;16.57,-43.48,;17.78,-40.99,;19.26,-41.44,;20.12,-40.16,;21.66,-40.11,;19.18,-38.94,;17.73,-39.47,;9.89,-48.88,;16.55,-51.17,;17.88,-50.4,;17.88,-48.87,;19.22,-51.17,)| Show InChI InChI=1S/C41H50FN7O4S/c1-26-21-49(22-27(2)44-26)23-29-7-12-36(31(17-29)24-48-13-15-52-16-14-48)30-5-4-6-35(18-30)53-40-37(19-32(42)20-43-40)38(50)46-33-8-10-34(11-9-33)47-39(51)41-45-28(3)25-54-41/h4-7,12,17-20,25-27,33-34,44H,8-11,13-16,21-24H2,1-3H3,(H,46,50)(H,47,51)/t26-,27+,33-,34+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017301
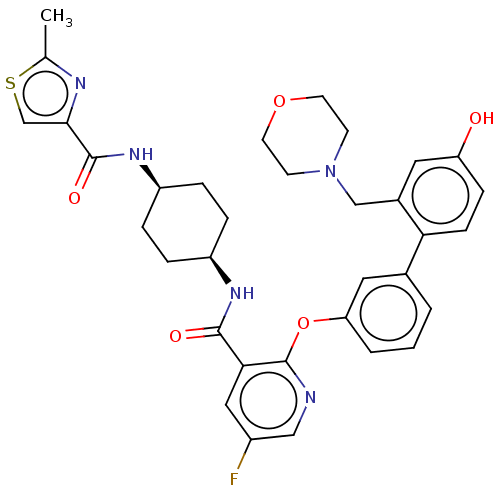
(CHEMBL3287988)Show SMILES Cc1nc(cs1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:12.16,9.9,(23.21,-22.73,;21.67,-22.78,;20.81,-24.05,;19.33,-23.61,;19.28,-22.09,;20.73,-21.56,;18.11,-24.55,;18.11,-26.09,;16.78,-23.78,;15.44,-24.55,;15.44,-26.09,;14.11,-26.86,;12.77,-26.09,;12.77,-24.55,;14.11,-23.78,;11.44,-26.86,;10.11,-26.1,;10.1,-24.56,;8.77,-26.87,;7.44,-26.11,;6.11,-26.88,;4.78,-26.11,;6.11,-28.42,;7.44,-29.19,;8.78,-28.42,;10.11,-29.19,;10.12,-30.73,;8.78,-31.5,;8.78,-33.04,;10.12,-33.81,;11.46,-33.03,;11.45,-31.49,;12.79,-33.79,;12.8,-35.33,;14.13,-36.09,;15.46,-35.31,;16.8,-36.07,;15.45,-33.77,;14.11,-33.01,;14.1,-31.47,;15.43,-30.69,;16.76,-31.45,;18.09,-30.67,;18.08,-29.13,;16.73,-28.37,;15.41,-29.15,)| Show InChI InChI=1S/C34H36FN5O5S/c1-21-37-31(20-46-21)33(43)39-26-7-5-25(6-8-26)38-32(42)30-17-24(35)18-36-34(30)45-28-4-2-3-22(16-28)29-10-9-27(41)15-23(29)19-40-11-13-44-14-12-40/h2-4,9-10,15-18,20,25-26,41H,5-8,11-14,19H2,1H3,(H,38,42)(H,39,43)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017314
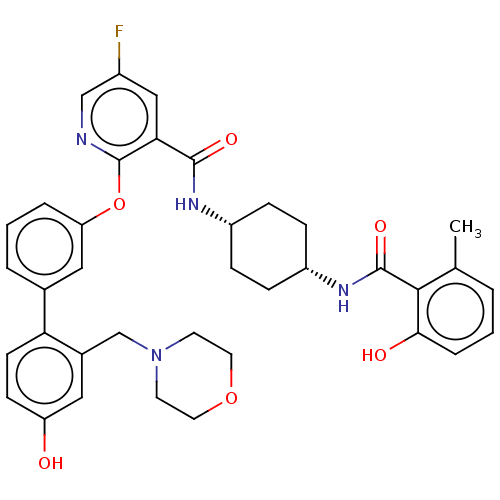
(CHEMBL3288000)Show SMILES Cc1cccc(O)c1C(=O)N[C@@H]1CC[C@@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:11.11,14.18,(36.27,-27.14,;36.28,-25.6,;37.61,-24.84,;37.61,-23.3,;36.27,-22.53,;34.95,-23.3,;33.61,-22.54,;34.95,-24.83,;33.62,-25.6,;33.62,-27.14,;32.29,-24.82,;30.94,-25.6,;29.61,-24.82,;28.27,-25.6,;28.27,-27.13,;29.61,-27.91,;30.94,-27.14,;26.94,-27.91,;25.6,-27.14,;25.6,-25.6,;24.27,-27.92,;22.93,-27.16,;21.6,-27.92,;20.26,-27.16,;21.59,-29.47,;22.94,-30.25,;24.28,-29.47,;25.61,-30.24,;25.61,-31.78,;24.28,-32.55,;24.28,-34.09,;25.62,-34.87,;26.96,-34.08,;26.95,-32.54,;28.29,-34.84,;28.3,-36.39,;29.63,-37.15,;30.97,-36.38,;32.31,-37.13,;30.95,-34.82,;29.62,-34.06,;29.6,-32.52,;30.93,-31.74,;32.27,-32.51,;33.59,-31.74,;33.59,-30.2,;32.25,-29.43,;30.92,-30.21,)| Show InChI InChI=1S/C37H39FN4O6/c1-23-4-2-7-33(44)34(23)36(46)41-28-10-8-27(9-11-28)40-35(45)32-20-26(38)21-39-37(32)48-30-6-3-5-24(19-30)31-13-12-29(43)18-25(31)22-42-14-16-47-17-15-42/h2-7,12-13,18-21,27-28,43-44H,8-11,14-17,22H2,1H3,(H,40,45)(H,41,46)/t27-,28+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017298
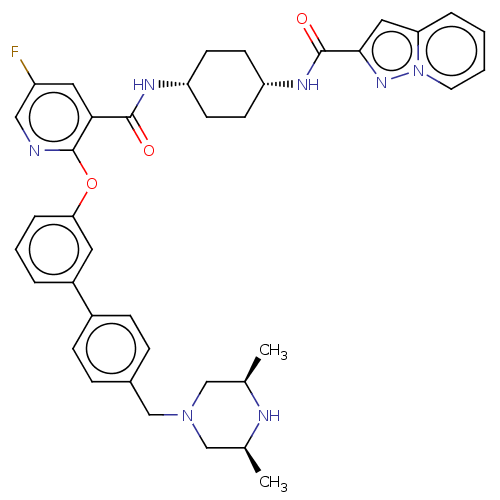
(CHEMBL3288010)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc4ccccn4n3)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,47.53,1.0,(29.15,-21.92,;30.48,-21.15,;30.48,-19.62,;31.81,-18.86,;31.8,-17.32,;30.47,-16.56,;29.13,-17.33,;27.8,-16.57,;27.79,-15.03,;29.12,-14.25,;30.45,-15.01,;26.45,-14.26,;25.12,-15.05,;23.78,-14.28,;23.78,-12.73,;25.11,-11.97,;25.11,-10.42,;23.77,-9.65,;22.44,-10.43,;21.09,-9.65,;21.09,-8.1,;19.76,-7.34,;22.43,-7.34,;23.77,-8.1,;25.1,-7.32,;25.1,-5.78,;26.44,-8.09,;27.77,-7.31,;29.11,-8.09,;30.44,-7.32,;30.45,-5.78,;29.11,-5,;27.77,-5.78,;31.79,-5,;33.12,-5.78,;33.12,-7.32,;34.36,-4.86,;34.34,-3.33,;35.79,-2.83,;36.4,-1.43,;37.92,-1.24,;38.84,-2.48,;38.23,-3.89,;36.71,-4.07,;35.83,-5.32,;26.45,-12.73,;33.14,-19.6,;33.14,-21.14,;34.48,-21.91,;31.82,-21.92,)| Show InChI InChI=1S/C39H42FN7O3/c1-25-22-46(23-26(2)42-25)24-27-9-11-28(12-10-27)29-6-5-8-34(18-29)50-39-35(19-30(40)21-41-39)37(48)43-31-13-15-32(16-14-31)44-38(49)36-20-33-7-3-4-17-47(33)45-36/h3-12,17-21,25-26,31-32,42H,13-16,22-24H2,1-2H3,(H,43,48)(H,44,49)/t25-,26+,31-,32+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50017295

(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat PDE4B |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017324
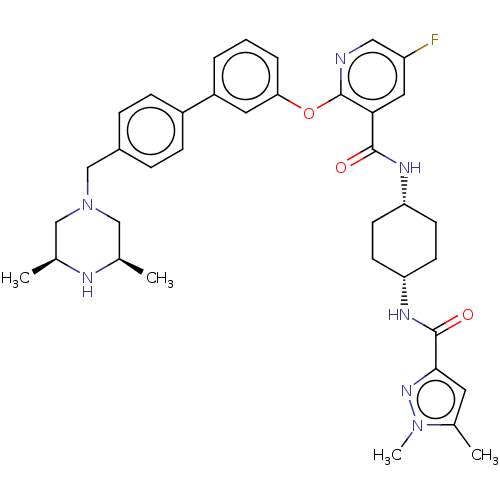
(CHEMBL3288012)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:1.0,45.50,30.35,27.28,(21.25,-53.01,;19.92,-52.24,;18.6,-53.01,;17.27,-52.24,;15.95,-53.02,;14.62,-52.26,;13.28,-53.03,;11.95,-52.27,;11.94,-50.73,;13.26,-49.95,;14.6,-50.71,;10.61,-49.96,;9.27,-50.75,;7.93,-49.97,;7.93,-48.43,;9.26,-47.67,;9.26,-46.12,;7.93,-45.35,;6.59,-46.13,;5.25,-45.35,;5.25,-43.81,;3.91,-43.04,;6.58,-43.04,;7.92,-43.8,;9.25,-43.03,;9.25,-41.48,;10.59,-43.79,;11.92,-43.02,;13.26,-43.79,;14.59,-43.02,;14.59,-41.48,;13.26,-40.71,;11.92,-41.48,;15.94,-40.7,;17.27,-41.48,;17.27,-43.02,;18.49,-40.53,;18.44,-39.01,;19.89,-38.48,;20.32,-37.01,;20.83,-39.7,;22.36,-39.65,;19.97,-40.98,;10.6,-48.42,;17.26,-50.71,;18.59,-49.94,;18.59,-48.41,;19.92,-50.71,)| Show InChI InChI=1S/C37H44FN7O3/c1-23-20-45(21-24(2)40-23)22-26-8-10-27(11-9-26)28-6-5-7-32(17-28)48-37-33(18-29(38)19-39-37)35(46)41-30-12-14-31(15-13-30)42-36(47)34-16-25(3)44(4)43-34/h5-11,16-19,23-24,30-31,40H,12-15,20-22H2,1-4H3,(H,41,46)(H,42,47)/t23-,24+,30-,31+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017327
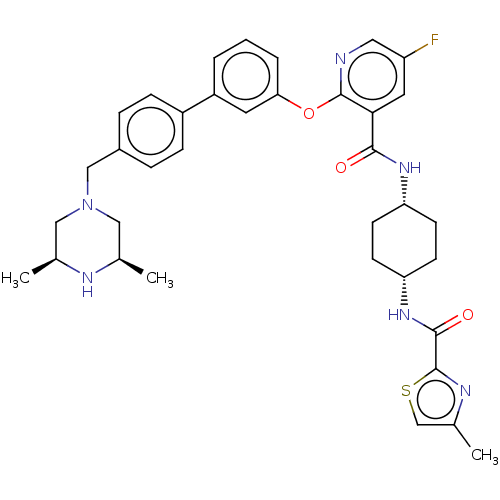
(CHEMBL3288016)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,1.0,44.49,(10.36,-38.26,;11.69,-37.49,;11.68,-35.96,;13.01,-35.19,;13,-33.66,;11.67,-32.9,;10.33,-33.67,;9,-32.91,;8.99,-31.37,;10.32,-30.59,;11.65,-31.35,;7.66,-30.6,;6.32,-31.39,;4.98,-30.62,;4.98,-29.08,;6.32,-28.31,;6.32,-26.77,;4.98,-26,;3.64,-26.77,;2.3,-26,;2.3,-24.45,;.97,-23.68,;3.64,-23.68,;4.98,-24.44,;6.31,-23.67,;6.3,-22.13,;7.64,-24.43,;8.97,-23.66,;10.32,-24.43,;11.64,-23.67,;11.64,-22.13,;10.31,-21.35,;8.97,-22.13,;12.99,-21.35,;14.32,-22.13,;14.32,-23.67,;15.54,-21.18,;17.02,-21.62,;17.88,-20.35,;19.42,-20.3,;16.94,-19.13,;15.49,-19.65,;7.65,-29.07,;14.34,-35.94,;14.35,-37.48,;15.68,-38.25,;13.02,-38.25,)| Show InChI InChI=1S/C36H41FN6O3S/c1-22-18-43(19-23(2)39-22)20-25-7-9-26(10-8-25)27-5-4-6-31(15-27)46-35-32(16-28(37)17-38-35)33(44)41-29-11-13-30(14-12-29)42-34(45)36-40-24(3)21-47-36/h4-10,15-17,21-23,29-30,39H,11-14,18-20H2,1-3H3,(H,41,44)(H,42,45)/t22-,23+,29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017311
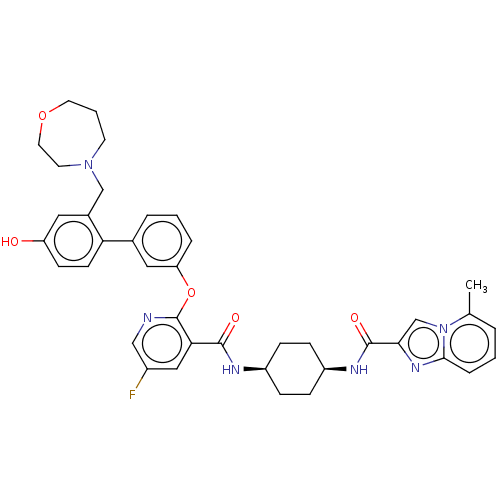
(CHEMBL3287997)Show SMILES Cc1cccc2nc(cn12)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCCOCC1 |r,wU:13.14,16.21,(37.17,-36.87,;38.11,-38.09,;39.63,-37.87,;40.58,-39.09,;39.99,-40.51,;38.48,-40.72,;37.62,-41.99,;36.14,-41.54,;36.09,-40.02,;37.54,-39.5,;34.92,-42.49,;34.92,-44.03,;33.59,-41.72,;32.25,-42.49,;32.24,-44.04,;30.92,-44.8,;29.58,-44.03,;29.57,-42.5,;30.91,-41.72,;28.24,-44.8,;26.91,-44.04,;26.9,-42.5,;25.58,-44.81,;24.24,-44.05,;22.91,-44.82,;21.57,-44.05,;22.9,-46.36,;24.25,-47.14,;25.58,-46.36,;26.92,-47.14,;26.92,-48.68,;25.58,-49.44,;25.59,-50.98,;26.92,-51.76,;28.26,-50.97,;28.25,-49.43,;29.59,-51.73,;29.6,-53.28,;30.94,-54.04,;32.27,-53.26,;33.61,-54.02,;32.25,-51.71,;30.92,-50.95,;30.91,-49.41,;32.23,-48.64,;32.11,-47.11,;33.23,-46.06,;34.75,-46.27,;35.52,-47.59,;34.98,-49.03,;33.51,-49.5,)| Show InChI InChI=1S/C39H41FN6O5/c1-25-5-2-8-36-44-35(24-46(25)36)38(49)43-30-11-9-29(10-12-30)42-37(48)34-21-28(40)22-41-39(34)51-32-7-3-6-26(20-32)33-14-13-31(47)19-27(33)23-45-15-4-17-50-18-16-45/h2-3,5-8,13-14,19-22,24,29-30,47H,4,9-12,15-18,23H2,1H3,(H,42,48)(H,43,49)/t29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017306
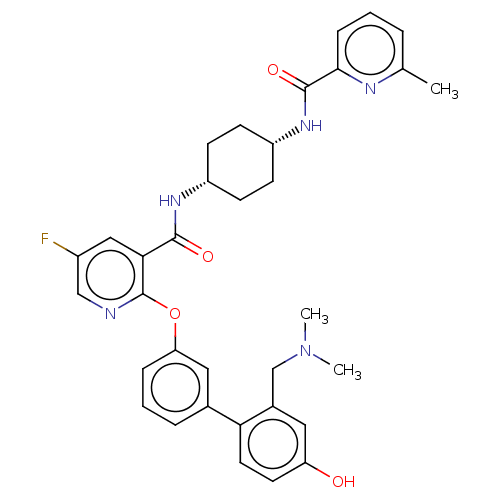
(CHEMBL3287993)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cccc(C)n2)c1 |r,wU:30.35,27.28,(21.27,-30.02,;19.93,-29.26,;19.92,-27.72,;18.6,-30.03,;18.61,-31.58,;19.95,-32.34,;19.97,-33.89,;21.31,-34.65,;18.63,-34.66,;17.3,-33.9,;17.29,-32.36,;15.96,-31.59,;14.62,-32.38,;13.28,-31.6,;13.28,-30.06,;14.61,-29.3,;14.61,-27.76,;13.28,-26.98,;11.94,-27.76,;10.6,-26.98,;10.6,-25.44,;9.26,-24.67,;11.94,-24.67,;13.27,-25.43,;14.6,-24.66,;14.6,-23.12,;15.94,-25.42,;17.27,-24.65,;18.61,-25.42,;19.94,-24.65,;19.94,-23.11,;18.61,-22.34,;17.27,-23.11,;21.29,-22.34,;22.62,-23.11,;22.62,-24.65,;23.95,-22.34,;23.95,-20.81,;25.27,-20.05,;26.61,-20.81,;26.6,-22.35,;27.93,-23.12,;25.27,-23.12,;15.95,-30.06,)| Show InChI InChI=1S/C34H36FN5O4/c1-21-6-4-9-31(37-21)33(43)39-26-12-10-25(11-13-26)38-32(42)30-18-24(35)19-36-34(30)44-28-8-5-7-22(17-28)29-15-14-27(41)16-23(29)20-40(2)3/h4-9,14-19,25-26,41H,10-13,20H2,1-3H3,(H,38,42)(H,39,43)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017302
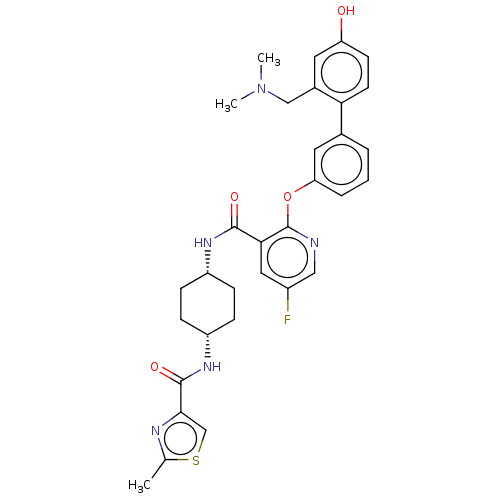
(CHEMBL3287989)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2csc(C)n2)c1 |r,wU:27.28,30.35,(14.8,-31.05,;13.47,-30.29,;13.45,-28.75,;12.14,-31.07,;12.15,-32.61,;13.49,-33.36,;13.5,-34.91,;14.84,-35.67,;12.17,-35.69,;10.84,-34.92,;10.83,-33.38,;9.5,-32.62,;8.16,-33.4,;6.82,-32.63,;6.82,-31.1,;8.16,-30.33,;8.15,-28.79,;6.82,-28.02,;5.48,-28.79,;4.15,-28.02,;4.15,-26.48,;2.82,-25.71,;5.48,-25.7,;6.81,-26.47,;8.15,-25.69,;8.14,-24.15,;9.48,-26.46,;10.81,-25.68,;12.15,-26.46,;13.48,-25.69,;13.48,-24.15,;12.15,-23.38,;10.81,-24.15,;14.82,-23.38,;16.15,-24.15,;16.15,-25.69,;17.37,-23.2,;17.32,-21.68,;18.77,-21.16,;19.71,-22.37,;21.25,-22.33,;18.85,-23.65,;9.49,-31.09,)| Show InChI InChI=1S/C32H34FN5O4S/c1-19-35-29(18-43-19)31(41)37-24-9-7-23(8-10-24)36-30(40)28-15-22(33)16-34-32(28)42-26-6-4-5-20(14-26)27-12-11-25(39)13-21(27)17-38(2)3/h4-6,11-16,18,23-24,39H,7-10,17H2,1-3H3,(H,36,40)(H,37,41)/t23-,24+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017333
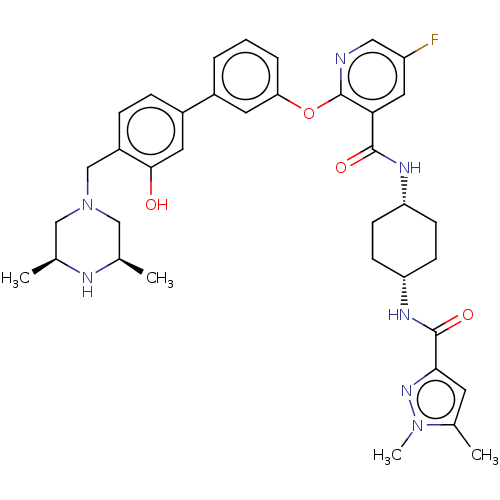
(CHEMBL3288022)Show SMILES C[C@H]1CN(Cc2ccc(cc2O)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:31.36,28.29,46.51,1.0,(19.64,-20.1,;18.31,-19.33,;16.99,-20.1,;15.66,-19.33,;14.33,-20.11,;13,-19.35,;11.67,-20.12,;10.33,-19.36,;10.33,-17.82,;11.65,-17.04,;12.99,-17.8,;14.31,-17.02,;8.99,-17.05,;7.65,-17.84,;6.32,-17.06,;6.32,-15.52,;7.65,-14.76,;7.65,-13.22,;6.31,-12.44,;4.97,-13.22,;3.63,-12.44,;3.63,-10.9,;2.3,-10.13,;4.97,-10.13,;6.31,-10.89,;7.64,-10.12,;7.63,-8.58,;8.98,-10.88,;10.31,-10.11,;11.65,-10.88,;12.98,-10.11,;12.98,-8.57,;11.64,-7.8,;10.3,-8.57,;14.32,-7.8,;15.66,-8.57,;15.66,-10.11,;16.87,-7.62,;16.82,-6.1,;18.27,-5.58,;18.7,-4.1,;19.22,-6.79,;20.76,-6.74,;18.35,-8.07,;8.98,-15.52,;15.64,-17.8,;16.97,-17.03,;16.97,-15.5,;18.31,-17.8,)| Show InChI InChI=1S/C37H44FN7O4/c1-22-19-45(20-23(2)40-22)21-27-9-8-26(16-34(27)46)25-6-5-7-31(15-25)49-37-32(17-28(38)18-39-37)35(47)41-29-10-12-30(13-11-29)42-36(48)33-14-24(3)44(4)43-33/h5-9,14-18,22-23,29-30,40,46H,10-13,19-21H2,1-4H3,(H,41,47)(H,42,48)/t22-,23+,29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017315
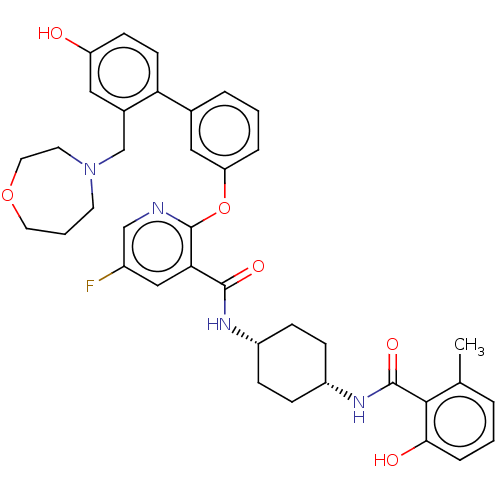
(CHEMBL3288001)Show SMILES Cc1cccc(O)c1C(=O)N[C@@H]1CC[C@@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCCOCC1 |r,wU:11.11,14.18,(55.81,-27.02,;55.81,-25.48,;57.14,-24.72,;57.14,-23.18,;55.8,-22.41,;54.48,-23.18,;53.15,-22.42,;54.49,-24.71,;53.16,-25.48,;53.16,-27.02,;51.82,-24.7,;50.48,-25.48,;49.14,-24.7,;47.8,-25.48,;47.81,-27.02,;49.15,-27.79,;50.48,-27.02,;46.47,-27.79,;45.14,-27.03,;45.13,-25.48,;43.8,-27.8,;42.47,-27.04,;41.13,-27.81,;39.8,-27.04,;41.13,-29.35,;42.47,-30.13,;43.81,-29.35,;45.15,-30.12,;45.15,-31.67,;43.81,-32.43,;43.81,-33.97,;45.15,-34.75,;46.49,-33.96,;46.48,-32.42,;47.83,-34.73,;47.83,-36.27,;49.17,-37.03,;50.5,-36.26,;51.84,-37.02,;50.49,-34.71,;49.15,-33.95,;49.14,-32.4,;50.47,-31.63,;50.34,-30.1,;51.46,-29.04,;52.99,-29.25,;53.76,-30.58,;53.21,-32.02,;51.74,-32.48,)| Show InChI InChI=1S/C38H41FN4O6/c1-24-5-2-8-34(45)35(24)37(47)42-29-11-9-28(10-12-29)41-36(46)33-21-27(39)22-40-38(33)49-31-7-3-6-25(20-31)32-14-13-30(44)19-26(32)23-43-15-4-17-48-18-16-43/h2-3,5-8,13-14,19-22,28-29,44-45H,4,9-12,15-18,23H2,1H3,(H,41,46)(H,42,47)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017336
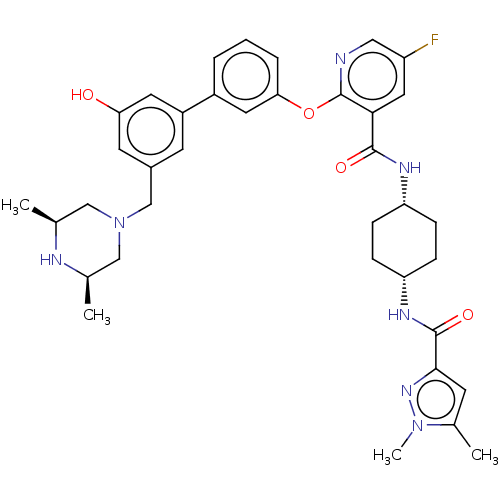
(CHEMBL3288025)Show SMILES C[C@H]1CN(Cc2cc(O)cc(c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:46.51,1.0,31.36,28.29,(42.43,-41.61,;42.45,-40.08,;41.13,-39.3,;41.14,-37.77,;39.79,-37.06,;38.46,-37.83,;38.47,-39.39,;37.14,-40.16,;37.15,-41.69,;35.81,-39.4,;35.8,-37.85,;37.12,-37.07,;34.46,-37.09,;33.13,-37.88,;31.79,-37.1,;31.79,-35.56,;33.12,-34.8,;33.12,-33.25,;31.79,-32.48,;30.45,-33.26,;29.1,-32.48,;29.11,-30.93,;27.77,-30.17,;30.44,-30.17,;31.78,-30.93,;33.11,-30.16,;33.11,-28.61,;34.45,-30.92,;35.78,-30.15,;37.12,-30.92,;38.45,-30.15,;38.45,-28.61,;37.12,-27.83,;35.78,-28.61,;39.8,-27.83,;41.13,-28.61,;41.13,-30.15,;42.35,-27.66,;42.3,-26.13,;43.75,-25.61,;44.18,-24.14,;44.69,-26.83,;46.23,-26.78,;43.83,-28.11,;34.46,-35.55,;42.47,-37,;43.79,-37.79,;45.13,-37.03,;43.78,-39.32,)| Show InChI InChI=1S/C37H44FN7O4/c1-22-19-45(20-23(2)40-22)21-25-13-27(15-31(46)14-25)26-6-5-7-32(16-26)49-37-33(17-28(38)18-39-37)35(47)41-29-8-10-30(11-9-29)42-36(48)34-12-24(3)44(4)43-34/h5-7,12-18,22-23,29-30,40,46H,8-11,19-21H2,1-4H3,(H,41,47)(H,42,48)/t22-,23+,29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017297

(CHEMBL3288009)Show SMILES Cc1cccc2nc(cn12)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(CN2CCC(CC2)N2CCCC2)cc1 |r,wU:13.14,16.21,(20.88,-1.83,;21.8,-3.06,;23.32,-2.87,;24.25,-4.11,;23.64,-5.52,;22.11,-5.7,;21.23,-6.95,;19.76,-6.49,;19.74,-4.96,;21.2,-4.46,;18.53,-7.41,;18.53,-8.95,;17.19,-6.63,;15.85,-7.41,;15.84,-8.95,;14.51,-9.72,;13.17,-8.95,;13.17,-7.41,;14.51,-6.64,;11.84,-9.72,;10.5,-8.96,;10.49,-7.41,;9.17,-9.73,;7.83,-8.97,;6.49,-9.74,;5.15,-8.97,;6.49,-11.29,;7.83,-12.06,;9.17,-11.29,;10.51,-12.06,;10.51,-13.6,;9.17,-14.37,;9.17,-15.91,;10.51,-16.69,;11.85,-15.9,;11.84,-14.36,;13.19,-16.67,;13.19,-18.21,;14.53,-18.97,;15.87,-18.2,;17.2,-18.96,;17.21,-20.5,;15.88,-21.26,;15.88,-22.79,;17.22,-23.56,;18.55,-22.79,;18.54,-21.25,;17.21,-25.09,;15.97,-25.99,;16.44,-27.46,;17.98,-27.45,;18.45,-25.99,;15.85,-16.65,;14.51,-15.89,)| Show InChI InChI=1S/C43H48FN7O3/c1-29-6-4-9-40-48-39(28-51(29)40)42(53)47-35-16-14-34(15-17-35)46-41(52)38-25-33(44)26-45-43(38)54-37-8-5-7-32(24-37)31-12-10-30(11-13-31)27-49-22-18-36(19-23-49)50-20-2-3-21-50/h4-13,24-26,28,34-36H,2-3,14-23,27H2,1H3,(H,46,52)(H,47,53)/t34-,35+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017339
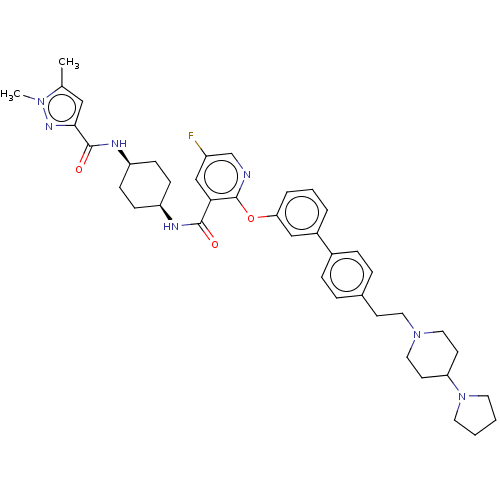
(CHEMBL3288028)Show SMILES Cc1cc(nn1C)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(CCN2CCC(CC2)N2CCCC2)cc1 |r,wU:10.10,13.17,(20.81,-18.66,;20.38,-20.13,;18.93,-20.66,;18.98,-22.18,;20.46,-22.63,;21.33,-21.35,;22.87,-21.3,;17.76,-23.13,;17.76,-24.67,;16.43,-22.36,;15.08,-23.13,;15.08,-24.67,;13.75,-25.44,;12.41,-24.67,;12.4,-23.13,;13.74,-22.36,;11.08,-25.44,;9.74,-24.68,;9.73,-23.14,;8.41,-25.45,;7.07,-24.69,;5.73,-25.46,;4.4,-24.69,;5.73,-27.01,;7.07,-27.78,;8.41,-27.01,;9.75,-27.78,;9.75,-29.32,;8.41,-30.09,;8.42,-31.63,;9.75,-32.4,;11.09,-31.62,;11.08,-30.08,;12.43,-32.38,;12.43,-33.93,;13.77,-34.69,;15.1,-33.91,;16.44,-34.67,;17.77,-33.9,;19.1,-34.66,;19.1,-36.19,;20.42,-36.95,;21.75,-36.19,;21.75,-34.65,;20.41,-33.88,;23.08,-36.95,;23.24,-38.48,;24.74,-38.8,;25.51,-37.46,;24.47,-36.33,;15.09,-32.36,;13.75,-31.6,)| Show InChI InChI=1S/C41H50FN7O3/c1-28-24-38(46-47(28)2)40(51)45-34-14-12-33(13-15-34)44-39(50)37-26-32(42)27-43-41(37)52-36-7-5-6-31(25-36)30-10-8-29(9-11-30)16-21-48-22-17-35(18-23-48)49-19-3-4-20-49/h5-11,24-27,33-35H,3-4,12-23H2,1-2H3,(H,44,50)(H,45,51)/t33-,34+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
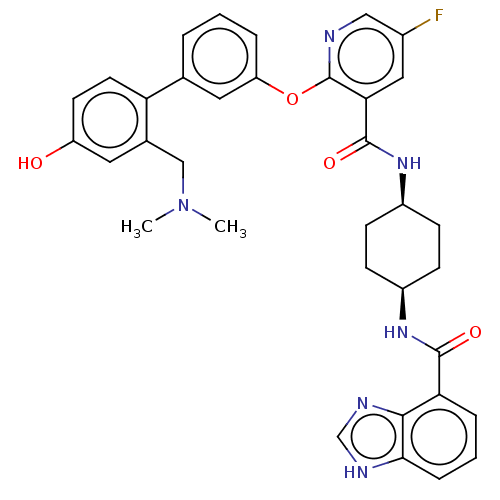
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017321
(CHEMBL3288006)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@H]2CC[C@H](CC2)NC(=O)c2cccc3[nH]cnc23)c1 |r,wU:30.35,27.28,(14.62,-29.31,;13.28,-28.54,;13.27,-27,;11.95,-29.32,;11.97,-30.86,;13.31,-31.62,;13.32,-33.17,;14.66,-33.93,;11.98,-33.95,;10.65,-33.19,;10.64,-31.64,;9.31,-30.88,;7.97,-31.66,;6.63,-30.89,;6.63,-29.35,;7.96,-28.58,;7.96,-27.04,;6.63,-26.26,;5.29,-27.04,;3.94,-26.26,;3.95,-24.72,;2.61,-23.95,;5.28,-23.95,;6.62,-24.71,;7.95,-23.94,;7.95,-22.4,;9.29,-24.7,;10.62,-23.93,;10.62,-22.39,;11.96,-21.62,;13.3,-22.39,;13.29,-23.93,;11.97,-24.7,;14.64,-21.62,;15.97,-22.39,;15.97,-23.93,;17.31,-21.62,;17.3,-20.09,;18.62,-19.32,;19.96,-20.09,;19.96,-21.63,;21.09,-22.66,;20.47,-24.05,;18.94,-23.89,;18.63,-22.39,;9.3,-29.34,)| Show InChI InChI=1S/C35H35FN6O4/c1-42(2)19-22-15-26(43)13-14-28(22)21-5-3-6-27(16-21)46-35-30(17-23(36)18-37-35)34(45)41-25-11-9-24(10-12-25)40-33(44)29-7-4-8-31-32(29)39-20-38-31/h3-8,13-18,20,24-25,43H,9-12,19H2,1-2H3,(H,38,39)(H,40,44)(H,41,45)/t24-,25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
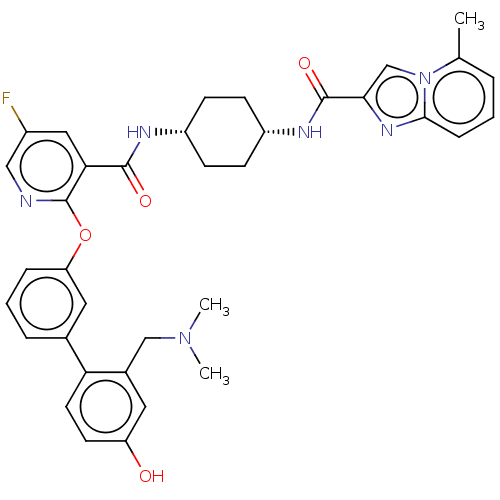
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017310
(CHEMBL3287996)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cn3c(C)cccc3n2)c1 |r,wU:30.35,27.28,(12.31,-47.81,;10.97,-47.04,;10.96,-45.5,;9.64,-47.81,;9.66,-49.36,;10.99,-50.12,;11.01,-51.67,;12.35,-52.42,;9.67,-52.44,;8.34,-51.68,;8.33,-50.14,;7,-49.37,;5.66,-50.16,;4.32,-49.38,;4.32,-47.84,;5.66,-47.08,;5.65,-45.54,;4.32,-44.76,;2.98,-45.54,;1.64,-44.76,;1.64,-43.22,;.31,-42.45,;2.98,-42.45,;4.31,-43.21,;5.65,-42.44,;5.64,-40.9,;6.98,-43.2,;8.31,-42.43,;9.66,-43.2,;10.98,-42.44,;10.98,-40.89,;9.65,-40.12,;8.31,-40.9,;12.33,-40.12,;13.66,-40.89,;13.66,-42.43,;14.88,-39.94,;14.83,-38.42,;16.28,-37.9,;16.85,-36.48,;15.91,-35.27,;18.37,-36.27,;19.32,-37.49,;18.74,-38.91,;17.22,-39.11,;16.36,-40.39,;6.99,-47.84,)| Show InChI InChI=1S/C36H37FN6O4/c1-22-6-4-9-33-41-32(21-43(22)33)35(46)40-27-12-10-26(11-13-27)39-34(45)31-18-25(37)19-38-36(31)47-29-8-5-7-23(17-29)30-15-14-28(44)16-24(30)20-42(2)3/h4-9,14-19,21,26-27,44H,10-13,20H2,1-3H3,(H,39,45)(H,40,46)/t26-,27+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017319
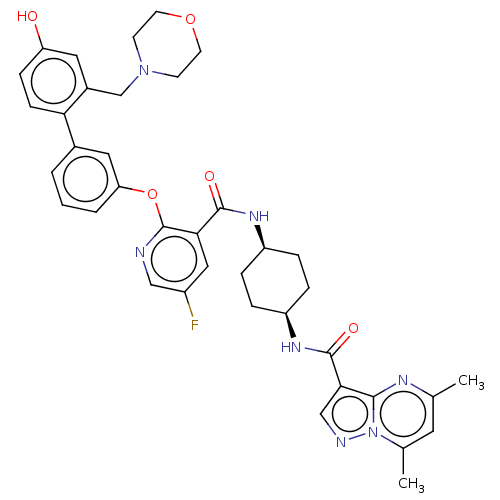
(CHEMBL3288005)Show SMILES Cc1cc(C)n2ncc(C(=O)N[C@H]3CC[C@H](CC3)NC(=O)c3cc(F)cnc3Oc3cccc(c3)-c3ccc(O)cc3CN3CCOCC3)c2n1 |r,wU:12.11,15.18,(20.6,-25.45,;20.12,-23.99,;21.15,-22.84,;20.67,-21.39,;21.7,-20.25,;19.18,-21.08,;18.41,-19.75,;16.91,-20.07,;16.75,-21.58,;15.42,-22.35,;15.42,-23.89,;14.08,-21.57,;12.74,-22.35,;12.74,-23.89,;11.41,-24.66,;10.07,-23.88,;10.06,-22.35,;11.4,-21.57,;8.73,-24.66,;7.4,-23.89,;7.39,-22.35,;6.06,-24.67,;4.73,-23.91,;3.39,-24.67,;2.05,-23.91,;3.39,-26.22,;4.73,-27,;6.07,-26.22,;7.4,-26.99,;7.41,-28.54,;6.07,-29.3,;6.07,-30.84,;7.41,-31.62,;8.75,-30.83,;8.74,-29.29,;10.08,-31.6,;10.09,-33.14,;11.43,-33.9,;12.76,-33.13,;14.1,-33.89,;12.75,-31.58,;11.41,-30.82,;11.4,-29.27,;12.73,-28.49,;14.06,-29.26,;15.39,-28.49,;15.39,-26.95,;14.05,-26.19,;12.71,-26.96,;18.15,-22.22,;18.62,-23.67,)| Show InChI InChI=1S/C38H40FN7O5/c1-23-16-24(2)46-35(42-23)34(21-41-46)37(49)44-29-8-6-28(7-9-29)43-36(48)33-19-27(39)20-40-38(33)51-31-5-3-4-25(18-31)32-11-10-30(47)17-26(32)22-45-12-14-50-15-13-45/h3-5,10-11,16-21,28-29,47H,6-9,12-15,22H2,1-2H3,(H,43,48)(H,44,49)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017312
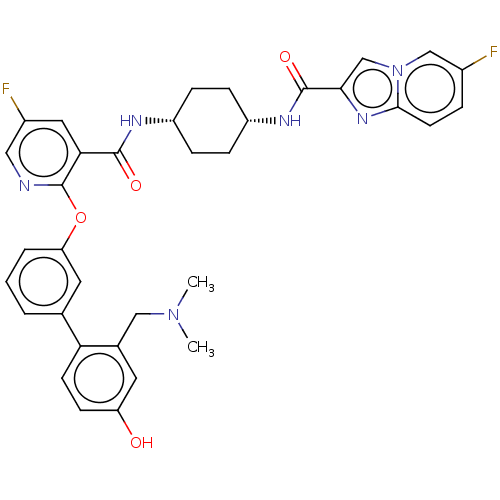
(CHEMBL3287998)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cn3cc(F)ccc3n2)c1 |r,wU:30.35,27.28,(39.15,-11.76,;37.81,-10.99,;37.8,-9.46,;36.48,-11.77,;36.5,-13.31,;37.83,-14.07,;37.85,-15.62,;39.19,-16.38,;36.51,-16.39,;35.18,-15.63,;35.17,-14.09,;33.84,-13.33,;32.5,-14.11,;31.17,-13.34,;31.16,-11.8,;32.5,-11.03,;32.5,-9.49,;31.16,-8.72,;29.82,-9.49,;28.48,-8.72,;28.48,-7.17,;27.15,-6.41,;29.82,-6.4,;31.16,-7.16,;32.49,-6.39,;32.48,-4.85,;33.82,-7.15,;35.15,-6.38,;36.5,-7.15,;37.82,-6.39,;37.82,-4.85,;36.49,-4.07,;35.15,-4.85,;39.17,-4.07,;40.5,-4.85,;40.5,-6.39,;41.72,-3.9,;41.67,-2.37,;43.12,-1.85,;43.69,-.44,;45.21,-.23,;45.78,1.21,;46.15,-1.44,;45.57,-2.86,;44.06,-3.07,;43.2,-4.34,;33.83,-11.79,)| Show InChI InChI=1S/C35H34F2N6O4/c1-42(2)18-22-14-27(44)11-12-29(22)21-4-3-5-28(15-21)47-35-30(16-24(37)17-38-35)33(45)39-25-7-9-26(10-8-25)40-34(46)31-20-43-19-23(36)6-13-32(43)41-31/h3-6,11-17,19-20,25-26,44H,7-10,18H2,1-2H3,(H,39,45)(H,40,46)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017305
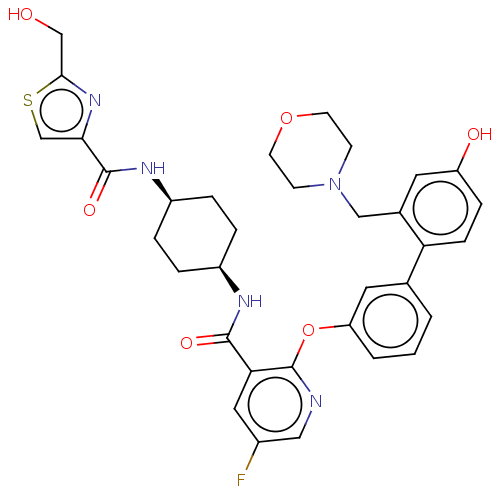
(CHEMBL3287992)Show SMILES OCc1nc(cs1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(O)cc1CN1CCOCC1 |r,wU:10.10,13.17,(22.02,-3.74,;21.3,-5.09,;19.76,-5.14,;18.89,-6.41,;17.41,-5.97,;17.36,-4.44,;18.81,-3.92,;16.2,-6.92,;16.2,-8.46,;14.86,-6.14,;13.52,-6.92,;13.52,-8.46,;12.19,-9.22,;10.85,-8.45,;10.85,-6.92,;12.18,-6.14,;9.52,-9.22,;8.18,-8.46,;8.18,-6.92,;6.85,-9.23,;5.52,-8.47,;4.18,-9.24,;2.85,-8.47,;4.18,-10.78,;5.52,-11.56,;6.86,-10.78,;8.19,-11.56,;8.2,-13.1,;6.86,-13.86,;6.86,-15.4,;8.2,-16.18,;9.54,-15.39,;9.53,-13.85,;10.87,-16.15,;10.88,-17.7,;12.21,-18.46,;13.54,-17.68,;14.88,-18.44,;13.53,-16.13,;12.19,-15.37,;12.18,-13.83,;13.51,-13.06,;14.84,-13.82,;16.17,-13.05,;16.16,-11.51,;14.83,-10.75,;13.49,-11.52,)| Show InChI InChI=1S/C34H36FN5O6S/c35-23-16-29(32(43)37-24-4-6-25(7-5-24)38-33(44)30-20-47-31(19-41)39-30)34(36-17-23)46-27-3-1-2-21(15-27)28-9-8-26(42)14-22(28)18-40-10-12-45-13-11-40/h1-3,8-9,14-17,20,24-25,41-42H,4-7,10-13,18-19H2,(H,37,43)(H,38,44)/t24-,25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017299
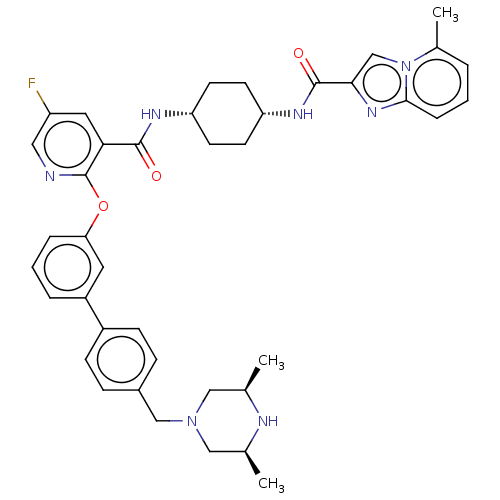
(CHEMBL3288011)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cn4c(C)cccc4n3)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,48.54,1.0,(14.61,-38.85,;15.94,-38.08,;15.93,-36.55,;17.26,-35.78,;17.25,-34.25,;15.92,-33.49,;14.59,-34.26,;13.25,-33.5,;13.24,-31.96,;14.57,-31.17,;15.91,-31.94,;11.91,-31.19,;10.57,-31.98,;9.23,-31.2,;9.23,-29.66,;10.57,-28.89,;10.56,-27.35,;9.23,-26.58,;7.89,-27.36,;6.54,-26.58,;6.55,-25.03,;5.21,-24.26,;7.88,-24.26,;9.22,-25.02,;10.55,-24.25,;10.55,-22.71,;11.89,-25.01,;13.22,-24.24,;14.57,-25.01,;15.9,-24.25,;15.9,-22.7,;14.56,-21.93,;13.22,-22.71,;17.24,-21.93,;18.58,-22.7,;18.58,-24.25,;19.81,-21.78,;19.79,-20.26,;21.25,-19.76,;21.85,-18.36,;20.93,-17.13,;23.37,-18.17,;24.29,-19.41,;23.68,-20.82,;22.16,-21,;21.28,-22.25,;11.9,-29.65,;18.59,-36.53,;18.6,-38.07,;19.93,-38.84,;17.27,-38.85,)| Show InChI InChI=1S/C40H44FN7O3/c1-25-21-47(22-26(2)43-25)23-28-10-12-29(13-11-28)30-7-5-8-34(18-30)51-40-35(19-31(41)20-42-40)38(49)44-32-14-16-33(17-15-32)45-39(50)36-24-48-27(3)6-4-9-37(48)46-36/h4-13,18-20,24-26,32-33,43H,14-17,21-23H2,1-3H3,(H,44,49)(H,45,50)/t25-,26+,32-,33+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017334
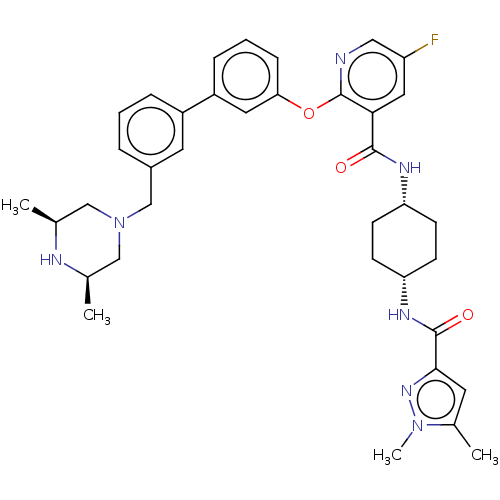
(CHEMBL3288023)Show SMILES C[C@H]1CN(Cc2cccc(c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:45.50,1.0,30.35,27.28,(39.54,-20.15,;39.6,-18.61,;38.31,-17.8,;38.37,-16.27,;37.04,-15.51,;35.71,-16.29,;35.73,-17.84,;34.39,-18.61,;33.06,-17.85,;33.05,-16.3,;34.38,-15.52,;31.72,-15.54,;30.38,-16.33,;29.04,-15.55,;29.04,-14.01,;30.38,-13.25,;30.37,-11.7,;29.04,-10.93,;27.7,-11.71,;26.36,-10.93,;26.36,-9.39,;25.02,-8.62,;27.7,-8.62,;29.03,-9.38,;30.36,-8.61,;30.36,-7.06,;31.7,-9.37,;33.03,-8.6,;34.37,-9.37,;35.7,-8.6,;35.7,-7.06,;34.37,-6.29,;33.03,-7.06,;37.05,-6.28,;38.38,-7.06,;38.38,-8.6,;39.6,-6.11,;39.55,-4.59,;41,-4.06,;41.43,-2.59,;41.94,-5.28,;43.49,-5.23,;41.08,-6.56,;31.71,-14,;39.72,-15.54,;41.02,-16.37,;42.38,-15.65,;40.96,-17.9,)| Show InChI InChI=1S/C37H44FN7O3/c1-23-20-45(21-24(2)40-23)22-26-7-5-8-27(16-26)28-9-6-10-32(17-28)48-37-33(18-29(38)19-39-37)35(46)41-30-11-13-31(14-12-30)42-36(47)34-15-25(3)44(4)43-34/h5-10,15-19,23-24,30-31,40H,11-14,20-22H2,1-4H3,(H,41,46)(H,42,47)/t23-,24+,30-,31+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017337
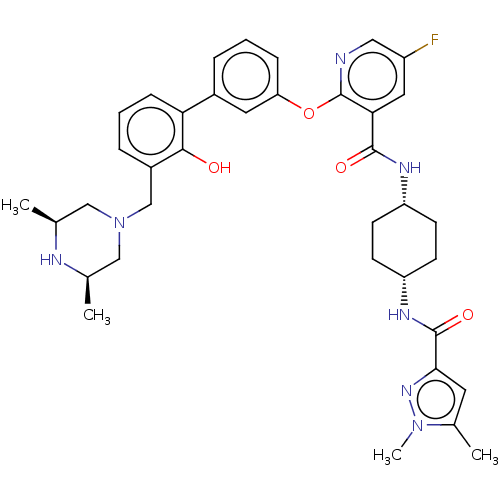
(CHEMBL3288026)Show SMILES C[C@H]1CN(Cc2cccc(c2O)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)n(C)n3)c2)C[C@@H](C)N1 |r,wU:46.51,1.0,31.36,28.29,(20.28,-61.8,;20.29,-60.27,;18.98,-59.49,;18.99,-57.96,;17.63,-57.24,;16.31,-58.02,;16.32,-59.57,;14.99,-60.35,;13.65,-59.59,;13.65,-58.04,;14.97,-57.26,;14.96,-55.73,;12.31,-57.28,;10.97,-58.06,;9.64,-57.29,;9.64,-55.75,;10.97,-54.98,;10.97,-53.44,;9.63,-52.67,;8.29,-53.44,;6.95,-52.67,;6.95,-51.12,;5.62,-50.35,;8.29,-50.35,;9.63,-51.11,;10.96,-50.34,;10.95,-48.8,;12.3,-51.1,;13.63,-50.33,;14.97,-51.1,;16.3,-50.34,;16.3,-48.79,;14.96,-48.02,;13.62,-48.8,;17.64,-48.02,;18.98,-48.79,;18.98,-50.34,;20.2,-47.84,;20.15,-46.32,;21.6,-45.8,;22.03,-44.32,;22.54,-47.02,;24.08,-46.97,;21.68,-48.29,;12.3,-55.74,;20.31,-57.19,;21.64,-57.97,;22.98,-57.21,;21.63,-59.51,)| Show InChI InChI=1S/C37H44FN7O4/c1-22-19-45(20-23(2)40-22)21-26-8-6-10-31(34(26)46)25-7-5-9-30(16-25)49-37-32(17-27(38)18-39-37)35(47)41-28-11-13-29(14-12-28)42-36(48)33-15-24(3)44(4)43-33/h5-10,15-18,22-23,28-29,40,46H,11-14,19-21H2,1-4H3,(H,41,47)(H,42,48)/t22-,23+,28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017328
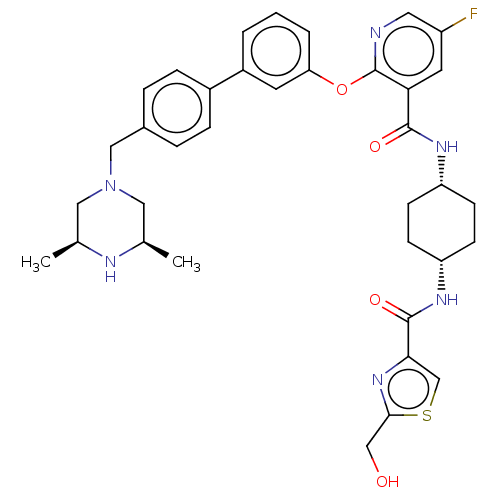
(CHEMBL3288017)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3csc(CO)n3)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,1.0,45.50,(31.33,-40.56,;32.66,-39.79,;32.65,-38.27,;33.98,-37.5,;33.97,-35.96,;32.63,-35.2,;31.3,-35.98,;29.97,-35.22,;29.96,-33.67,;31.28,-32.89,;32.62,-33.65,;28.62,-32.91,;27.29,-33.7,;25.95,-32.92,;25.95,-31.38,;27.28,-30.61,;27.28,-29.07,;25.95,-28.3,;24.61,-29.08,;23.27,-28.3,;23.27,-26.75,;21.93,-25.99,;24.61,-25.99,;25.94,-26.75,;27.27,-25.98,;27.27,-24.43,;28.61,-26.74,;29.94,-25.97,;31.28,-26.74,;32.61,-25.97,;32.61,-24.43,;31.28,-23.65,;29.94,-24.43,;33.95,-23.65,;35.29,-24.43,;35.29,-25.97,;36.51,-23.48,;36.46,-21.96,;37.91,-21.43,;38.85,-22.65,;40.39,-22.6,;41.2,-23.91,;37.99,-23.93,;28.62,-31.37,;35.31,-38.25,;35.32,-39.79,;36.65,-40.55,;33.99,-40.56,)| Show InChI InChI=1S/C36H41FN6O4S/c1-22-17-43(18-23(2)39-22)19-24-6-8-25(9-7-24)26-4-3-5-30(14-26)47-36-31(15-27(37)16-38-36)34(45)40-28-10-12-29(13-11-28)41-35(46)32-21-48-33(20-44)42-32/h3-9,14-16,21-23,28-29,39,44H,10-13,17-20H2,1-2H3,(H,40,45)(H,41,46)/t22-,23+,28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017326
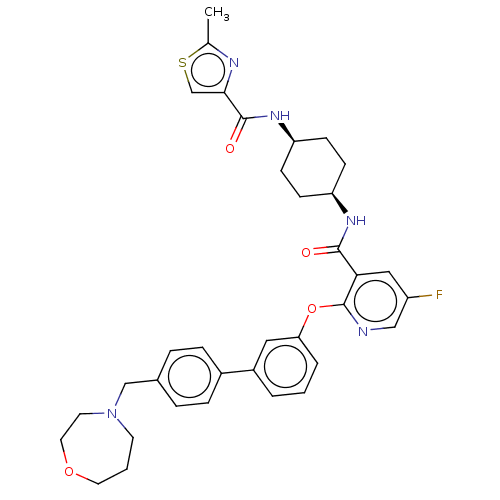
(CHEMBL3288015)Show SMILES Cc1nc(cs1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(CN2CCCOCC2)cc1 |r,wU:9.9,12.16,(49.63,.92,;48.09,.87,;47.23,-.41,;45.75,.04,;45.7,1.57,;47.15,2.09,;44.53,-.91,;44.53,-2.46,;43.2,-.14,;41.85,-.91,;41.85,-2.46,;40.52,-3.22,;39.18,-2.45,;39.18,-.92,;40.52,-.14,;37.85,-3.22,;36.51,-2.46,;36.51,-.92,;35.18,-3.23,;33.84,-2.47,;32.51,-3.24,;31.17,-2.47,;32.5,-4.79,;33.85,-5.56,;35.19,-4.79,;36.52,-5.56,;36.52,-7.1,;35.19,-7.87,;35.19,-9.41,;36.53,-10.18,;37.86,-9.4,;37.86,-7.86,;39.2,-10.16,;39.2,-11.7,;40.54,-12.46,;41.87,-11.69,;43.21,-12.45,;43.22,-13.99,;44.59,-14.64,;44.95,-16.14,;44.01,-17.35,;42.47,-17.35,;41.5,-16.16,;41.83,-14.66,;41.86,-10.14,;40.52,-9.38,)| Show InChI InChI=1S/C35H38FN5O4S/c1-23-38-32(22-46-23)34(43)40-29-12-10-28(11-13-29)39-33(42)31-19-27(36)20-37-35(31)45-30-5-2-4-26(18-30)25-8-6-24(7-9-25)21-41-14-3-16-44-17-15-41/h2,4-9,18-20,22,28-29H,3,10-17,21H2,1H3,(H,39,42)(H,40,43)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017331
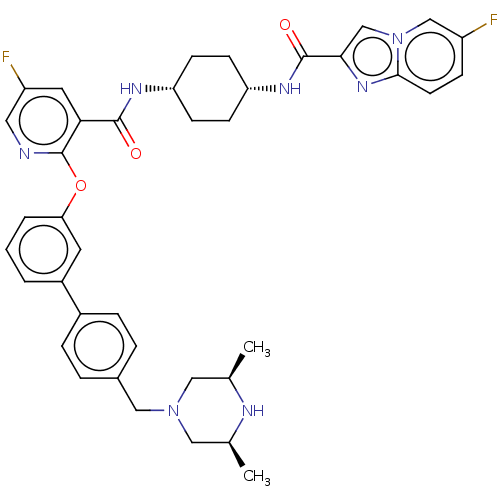
(CHEMBL3288020)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cn4cc(F)ccc4n3)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,1.0,48.54,(12.02,-41.43,;13.35,-40.66,;13.34,-39.13,;14.67,-38.36,;14.66,-36.83,;13.32,-36.07,;11.99,-36.84,;10.66,-36.08,;10.65,-34.54,;11.97,-33.76,;13.31,-34.52,;9.32,-33.77,;7.98,-34.56,;6.64,-33.79,;6.64,-32.25,;7.98,-31.48,;7.97,-29.94,;6.64,-29.17,;5.3,-29.94,;3.96,-29.17,;3.96,-27.62,;2.63,-26.85,;5.3,-26.85,;6.63,-27.61,;7.96,-26.84,;7.96,-25.3,;9.3,-27.6,;10.63,-26.83,;11.97,-27.6,;13.3,-26.84,;13.3,-25.3,;11.97,-24.52,;10.63,-25.3,;14.64,-24.52,;15.98,-25.3,;15.98,-26.84,;17.2,-24.35,;17.15,-22.82,;18.6,-22.3,;19.18,-20.88,;20.71,-20.67,;21.28,-19.24,;21.66,-21.89,;21.07,-23.32,;19.54,-23.53,;18.67,-24.79,;9.31,-32.24,;16,-39.11,;16,-40.65,;17.34,-41.41,;14.68,-41.42,)| Show InChI InChI=1S/C39H41F2N7O3/c1-24-19-47(20-25(2)43-24)21-26-6-8-27(9-7-26)28-4-3-5-33(16-28)51-39-34(17-30(41)18-42-39)37(49)44-31-11-13-32(14-12-31)45-38(50)35-23-48-22-29(40)10-15-36(48)46-35/h3-10,15-18,22-25,31-32,43H,11-14,19-21H2,1-2H3,(H,44,49)(H,45,50)/t24-,25+,31-,32+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017330
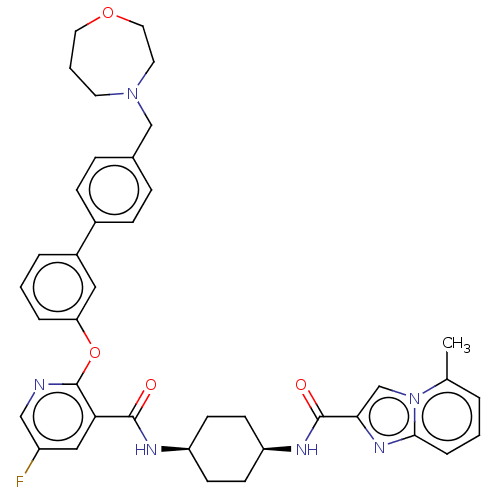
(CHEMBL3288019)Show SMILES Cc1cccc2nc(cn12)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1cccc(c1)-c1ccc(CN2CCCOCC2)cc1 |r,wU:13.14,16.21,(42.74,-.17,;43.68,-1.37,;45.21,-1.16,;46.16,-2.39,;45.58,-3.82,;44.05,-4.02,;43.18,-5.29,;41.7,-4.84,;41.65,-3.32,;43.1,-2.79,;40.48,-5.79,;40.48,-7.33,;39.15,-5.01,;37.81,-5.79,;37.81,-7.33,;36.48,-8.1,;35.14,-7.32,;35.13,-5.79,;36.47,-5.01,;33.81,-8.1,;32.47,-7.33,;32.46,-5.79,;31.14,-8.11,;29.8,-7.35,;28.47,-8.11,;27.13,-7.35,;28.47,-9.66,;29.81,-10.43,;31.14,-9.66,;32.48,-10.43,;32.48,-11.97,;31.15,-12.74,;31.15,-14.28,;32.49,-15.05,;33.82,-14.27,;33.81,-12.73,;35.16,-15.03,;35.16,-16.57,;36.5,-17.33,;37.83,-16.56,;39.16,-17.32,;39.17,-18.85,;40.55,-19.51,;40.9,-21,;39.96,-22.21,;38.43,-22.22,;37.46,-21.03,;37.79,-19.53,;37.82,-15.01,;36.48,-14.25,)| Show InChI InChI=1S/C39H41FN6O4/c1-26-5-2-8-36-44-35(25-46(26)36)38(48)43-32-15-13-31(14-16-32)42-37(47)34-22-30(40)23-41-39(34)50-33-7-3-6-29(21-33)28-11-9-27(10-12-28)24-45-17-4-19-49-20-18-45/h2-3,5-12,21-23,25,31-32H,4,13-20,24H2,1H3,(H,42,47)(H,43,48)/t31-,32+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017332
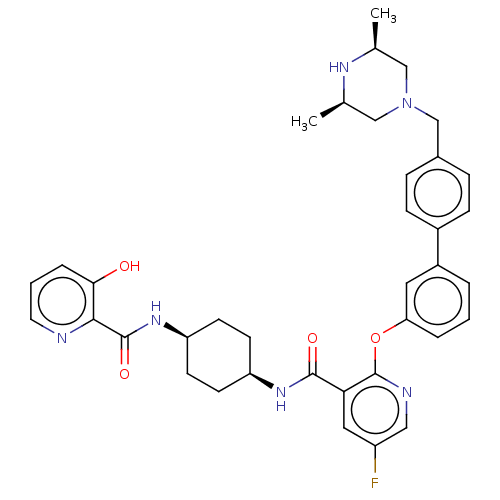
(CHEMBL3288021)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@H]3CC[C@H](CC3)NC(=O)c3ncccc3O)c2)C[C@@H](C)N1 |r,wU:30.35,27.28,1.0,45.50,(12.67,-41.64,;14,-40.87,;13.99,-39.34,;15.32,-38.58,;15.31,-37.04,;13.97,-36.28,;12.64,-37.05,;11.3,-36.29,;11.3,-34.75,;12.62,-33.97,;13.96,-34.73,;9.96,-33.98,;8.62,-34.77,;7.29,-34,;7.28,-32.45,;8.62,-31.69,;8.62,-30.14,;7.28,-29.37,;5.94,-30.15,;4.6,-29.37,;4.6,-27.82,;3.27,-27.06,;5.94,-27.06,;7.28,-27.82,;8.61,-27.05,;8.6,-25.5,;9.95,-27.81,;11.28,-27.04,;11.27,-25.5,;12.61,-24.72,;13.95,-25.5,;13.95,-27.04,;12.62,-27.81,;15.29,-24.72,;16.63,-25.5,;16.63,-27.04,;17.96,-24.73,;19.29,-25.51,;20.62,-24.74,;20.62,-23.21,;19.28,-22.44,;17.96,-23.21,;16.62,-22.44,;9.95,-32.45,;16.65,-39.33,;16.66,-40.87,;17.99,-41.63,;15.33,-41.64,)| Show InChI InChI=1S/C37H41FN6O4/c1-23-20-44(21-24(2)41-23)22-25-8-10-26(11-9-25)27-5-3-6-31(17-27)48-37-32(18-28(38)19-40-37)35(46)42-29-12-14-30(15-13-29)43-36(47)34-33(45)7-4-16-39-34/h3-11,16-19,23-24,29-30,41,45H,12-15,20-22H2,1-2H3,(H,42,46)(H,43,47)/t23-,24+,29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017317
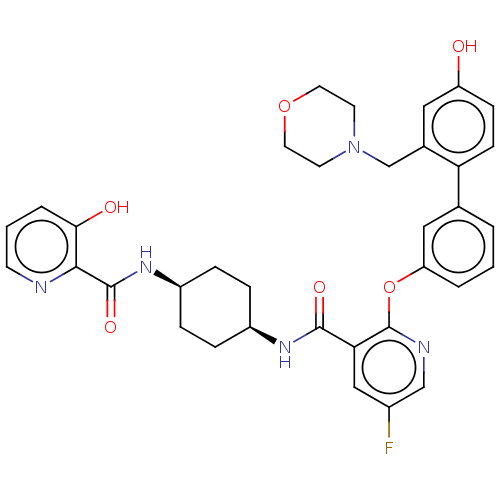
(CHEMBL3288003)Show SMILES Oc1ccc(c(CN2CCOCC2)c1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@H]2CC[C@H](CC2)NC(=O)c2ncccc2O)c1 |r,wU:33.39,30.32,(32.12,-20.38,;30.78,-19.62,;29.45,-20.4,;28.11,-19.64,;28.1,-18.09,;29.43,-17.31,;29.41,-15.77,;30.74,-14.99,;32.08,-15.76,;33.4,-14.99,;33.4,-13.45,;32.06,-12.69,;30.73,-13.46,;30.76,-18.07,;26.77,-17.33,;25.43,-18.12,;24.09,-17.34,;24.09,-15.8,;25.43,-15.04,;25.43,-13.49,;24.09,-12.72,;22.75,-13.5,;21.41,-12.72,;21.41,-11.18,;20.08,-10.41,;22.75,-10.41,;24.08,-11.17,;25.42,-10.4,;25.41,-8.85,;26.75,-11.16,;28.08,-10.39,;28.08,-8.85,;29.42,-8.08,;30.76,-8.85,;30.75,-10.39,;29.43,-11.16,;32.1,-8.07,;33.43,-8.85,;33.43,-10.39,;34.76,-8.08,;36.09,-8.86,;37.42,-8.09,;37.42,-6.55,;36.08,-5.79,;34.76,-6.55,;33.42,-5.79,;26.76,-15.79,)| Show InChI InChI=1S/C35H36FN5O6/c36-24-19-30(33(44)39-25-6-8-26(9-7-25)40-34(45)32-31(43)5-2-12-37-32)35(38-20-24)47-28-4-1-3-22(18-28)29-11-10-27(42)17-23(29)21-41-13-15-46-16-14-41/h1-5,10-12,17-20,25-26,42-43H,6-9,13-16,21H2,(H,39,44)(H,40,45)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017316
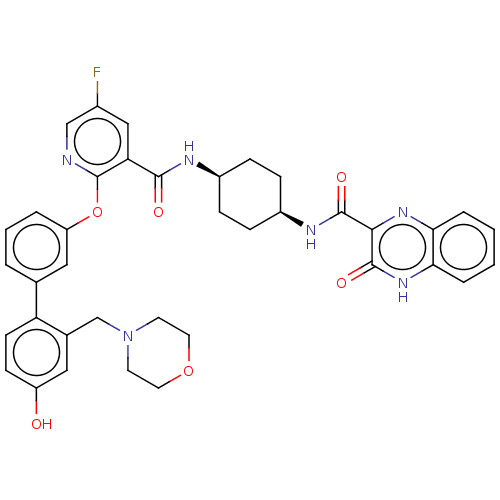
(CHEMBL3288002)Show SMILES Oc1ccc(c(CN2CCOCC2)c1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@H]2CC[C@H](CC2)NC(=O)c2nc3ccccc3[nH]c2=O)c1 |r,wU:33.39,30.32,(14.51,-17.07,;13.17,-16.31,;11.84,-17.09,;10.5,-16.33,;10.5,-14.78,;11.82,-14,;11.81,-12.46,;13.14,-11.69,;14.47,-12.45,;15.79,-11.68,;15.79,-10.14,;14.46,-9.38,;13.12,-10.15,;13.16,-14.76,;9.16,-14.02,;7.83,-14.81,;6.49,-14.03,;6.49,-12.49,;7.82,-11.73,;7.82,-10.19,;6.49,-9.41,;5.15,-10.19,;3.81,-9.41,;3.81,-7.87,;2.48,-7.1,;5.15,-7.1,;6.48,-7.86,;7.81,-7.09,;7.81,-5.55,;9.15,-7.85,;10.48,-7.08,;10.48,-5.55,;11.81,-4.77,;13.15,-5.54,;13.15,-7.09,;11.82,-7.85,;14.49,-4.77,;15.82,-5.54,;15.82,-7.09,;17.15,-4.78,;18.48,-5.55,;19.8,-4.79,;21.13,-5.57,;22.47,-4.8,;22.47,-3.25,;21.14,-2.48,;19.81,-3.25,;18.47,-2.48,;17.15,-3.25,;15.82,-2.49,;9.16,-12.48,)| Show InChI InChI=1S/C38H37FN6O6/c39-25-20-31(35(47)41-26-8-10-27(11-9-26)42-36(48)34-37(49)44-33-7-2-1-6-32(33)43-34)38(40-21-25)51-29-5-3-4-23(19-29)30-13-12-28(46)18-24(30)22-45-14-16-50-17-15-45/h1-7,12-13,18-21,26-27,46H,8-11,14-17,22H2,(H,41,47)(H,42,48)(H,44,49)/t26-,27+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017322
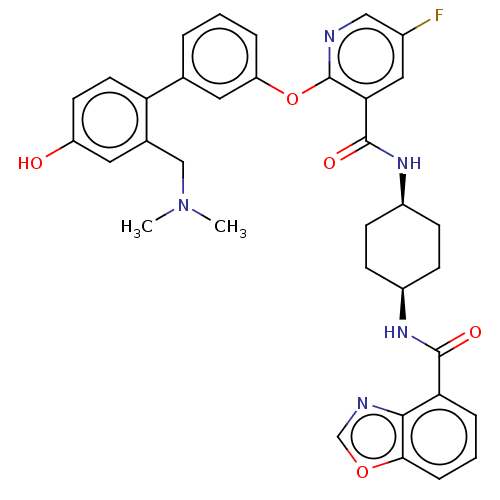
(CHEMBL3288007)Show SMILES CN(C)Cc1cc(O)ccc1-c1cccc(Oc2ncc(F)cc2C(=O)N[C@H]2CC[C@H](CC2)NC(=O)c2cccc3ocnc23)c1 |r,wU:30.35,27.28,(12.24,-28.55,;10.9,-27.78,;10.89,-26.25,;9.57,-28.56,;9.58,-30.1,;10.92,-30.86,;10.94,-32.42,;12.28,-33.17,;9.6,-33.19,;8.27,-32.43,;8.26,-30.88,;6.92,-30.12,;5.59,-30.91,;4.25,-30.13,;4.25,-28.59,;5.58,-27.82,;5.58,-26.28,;4.24,-25.51,;2.91,-26.29,;1.56,-25.51,;1.56,-23.96,;.23,-23.2,;2.9,-23.19,;4.24,-23.96,;5.57,-23.18,;5.57,-21.64,;6.91,-23.95,;8.24,-23.17,;8.24,-21.64,;9.58,-20.86,;10.91,-21.64,;10.91,-23.18,;9.58,-23.94,;12.26,-20.86,;13.59,-21.64,;13.59,-23.18,;14.92,-20.87,;14.92,-19.34,;16.24,-18.57,;17.58,-19.34,;17.58,-20.88,;18.71,-21.91,;18.08,-23.3,;16.56,-23.13,;16.25,-21.64,;6.92,-28.58,)| Show InChI InChI=1S/C35H34FN5O5/c1-41(2)19-22-15-26(42)13-14-28(22)21-5-3-6-27(16-21)46-35-30(17-23(36)18-37-35)34(44)40-25-11-9-24(10-12-25)39-33(43)29-7-4-8-31-32(29)38-20-45-31/h3-8,13-18,20,24-25,42H,9-12,19H2,1-2H3,(H,39,43)(H,40,44)/t24-,25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017325

(CHEMBL3288014)Show SMILES CN(C)Cc1ccc(cc1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2csc(C)n2)c1 |r,wU:29.34,26.27,(13.15,-16.41,;14.48,-15.64,;15.81,-16.39,;14.47,-14.11,;13.14,-13.35,;11.8,-14.12,;10.47,-13.36,;10.46,-11.82,;11.79,-11.04,;13.12,-11.8,;9.13,-11.06,;7.79,-11.84,;6.46,-11.07,;6.45,-9.53,;7.79,-8.76,;7.79,-7.22,;6.45,-6.45,;5.11,-7.22,;3.77,-6.45,;3.77,-4.9,;2.44,-4.14,;5.11,-4.14,;6.45,-4.9,;7.78,-4.13,;7.77,-2.58,;9.11,-4.89,;10.44,-4.12,;11.79,-4.89,;13.11,-4.12,;13.11,-2.58,;11.78,-1.81,;10.44,-2.58,;14.46,-1.81,;15.79,-2.58,;15.79,-4.12,;17.01,-1.63,;16.96,-.11,;18.41,.42,;19.35,-.8,;20.89,-.75,;18.49,-2.08,;9.12,-9.52,)| Show InChI InChI=1S/C32H34FN5O3S/c1-20-35-29(19-42-20)31(40)37-26-13-11-25(12-14-26)36-30(39)28-16-24(33)17-34-32(28)41-27-6-4-5-23(15-27)22-9-7-21(8-10-22)18-38(2)3/h4-10,15-17,19,25-26H,11-14,18H2,1-3H3,(H,36,39)(H,37,40)/t25-,26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017340
(CHEMBL3113734)Show SMILES Cc1ccc(O)c(c1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1ccc(F)c(F)c1 |r,wU:14.18,11.11,(24.27,-11.72,;24.28,-13.26,;25.62,-14.03,;25.62,-15.57,;24.29,-16.34,;24.28,-17.88,;22.96,-15.57,;22.95,-14.04,;21.63,-16.34,;21.63,-17.88,;20.29,-15.57,;18.96,-16.34,;18.96,-17.88,;17.63,-18.65,;16.29,-17.87,;16.28,-16.34,;17.62,-15.57,;14.96,-18.65,;13.62,-17.88,;13.62,-16.34,;12.29,-18.66,;10.95,-17.9,;9.63,-18.67,;8.29,-17.9,;9.62,-20.21,;10.96,-20.98,;12.3,-20.21,;13.63,-20.98,;13.63,-22.52,;12.3,-23.29,;12.3,-24.83,;13.64,-25.6,;13.64,-27.14,;14.97,-24.81,;16.31,-25.58,;14.96,-23.28,)| Show InChI InChI=1S/C26H24F3N3O4/c1-14-2-9-23(33)19(10-14)24(34)31-16-3-5-17(6-4-16)32-25(35)20-11-15(27)13-30-26(20)36-18-7-8-21(28)22(29)12-18/h2,7-13,16-17,33H,3-6H2,1H3,(H,31,34)(H,32,35)/t16-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017329
(CHEMBL3288018)Show SMILES CN(C)Cc1ccc(cc1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cn3c(C)cccc3n2)c1 |r,wU:29.34,26.27,(15.65,-19.05,;16.97,-18.28,;18.3,-19.05,;16.96,-16.75,;15.63,-15.99,;14.3,-16.76,;12.96,-16,;12.96,-14.46,;14.28,-13.68,;15.62,-14.44,;11.62,-13.7,;10.29,-14.48,;8.95,-13.71,;8.95,-12.17,;10.29,-11.41,;10.28,-9.87,;8.95,-9.09,;7.61,-9.87,;6.27,-9.09,;6.27,-7.55,;4.94,-6.78,;7.61,-6.78,;8.94,-7.54,;10.27,-6.77,;10.27,-5.23,;11.61,-7.53,;12.94,-6.76,;14.28,-7.53,;15.61,-6.77,;15.61,-5.23,;14.27,-4.45,;12.94,-5.23,;16.95,-4.45,;18.28,-5.23,;18.28,-6.77,;19.5,-4.28,;19.45,-2.76,;20.9,-2.23,;21.48,-.81,;20.54,.4,;23.01,-.6,;23.96,-1.83,;23.37,-3.26,;21.84,-3.46,;20.98,-4.72,;11.62,-12.16,)| Show InChI InChI=1S/C36H37FN6O3/c1-23-6-4-9-33-41-32(22-43(23)33)35(45)40-29-16-14-28(15-17-29)39-34(44)31-19-27(37)20-38-36(31)46-30-8-5-7-26(18-30)25-12-10-24(11-13-25)21-42(2)3/h4-13,18-20,22,28-29H,14-17,21H2,1-3H3,(H,39,44)(H,40,45)/t28-,29+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50017318
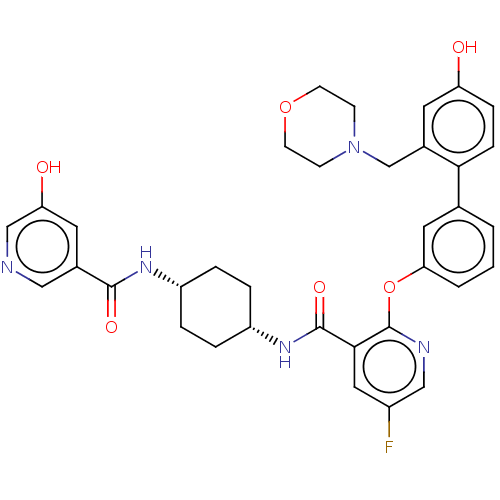
(CHEMBL3288004)Show SMILES Oc1ccc(c(CN2CCOCC2)c1)-c1cccc(Oc2ncc(F)cc2C(=O)N[C@@H]2CC[C@@H](CC2)NC(=O)c2cncc(O)c2)c1 |r,wU:33.39,30.32,(16.71,-34.22,;15.38,-33.46,;14.04,-34.24,;12.71,-33.47,;12.7,-31.93,;14.02,-31.15,;14.01,-29.61,;15.34,-28.83,;16.68,-29.6,;18,-28.83,;18,-27.29,;16.66,-26.52,;15.33,-27.3,;15.36,-31.91,;11.37,-31.17,;10.03,-31.95,;8.69,-31.18,;8.69,-29.64,;10.02,-28.87,;10.02,-27.33,;8.69,-26.56,;7.35,-27.33,;6.01,-26.56,;6.01,-25.01,;4.67,-24.24,;7.34,-24.24,;8.68,-25,;10.01,-24.23,;10.01,-22.69,;11.35,-24.99,;12.68,-24.22,;14.02,-24.99,;15.35,-24.23,;15.35,-22.68,;14.02,-21.91,;12.68,-22.69,;16.7,-21.91,;18.03,-22.68,;18.03,-24.23,;19.36,-21.92,;19.35,-20.39,;20.68,-19.62,;22.02,-20.39,;22.01,-21.93,;23.34,-22.7,;20.68,-22.69,;11.36,-29.63,)| Show InChI InChI=1S/C35H36FN5O6/c36-25-17-32(34(45)40-27-6-4-26(5-7-27)39-33(44)23-14-29(43)20-37-18-23)35(38-19-25)47-30-3-1-2-22(16-30)31-9-8-28(42)15-24(31)21-41-10-12-46-13-11-41/h1-3,8-9,14-20,26-27,42-43H,4-7,10-13,21H2,(H,39,44)(H,40,45)/t26-,27+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50017295

(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4A1A using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data