 Found 44 hits Enz. Inhib. hit(s) with all data for entry = 50044471
Found 44 hits Enz. Inhib. hit(s) with all data for entry = 50044471 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018507
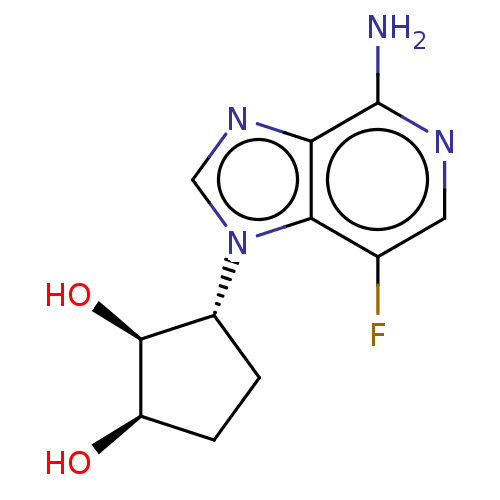
(CHEMBL3290657)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006218
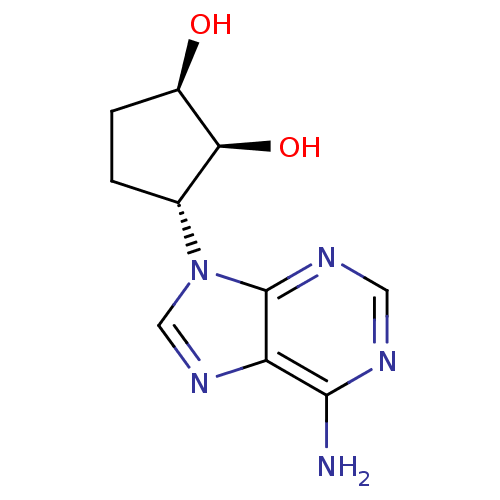
((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...)Show InChI InChI=1S/C10H13N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h3-6,8,16-17H,1-2H2,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018501
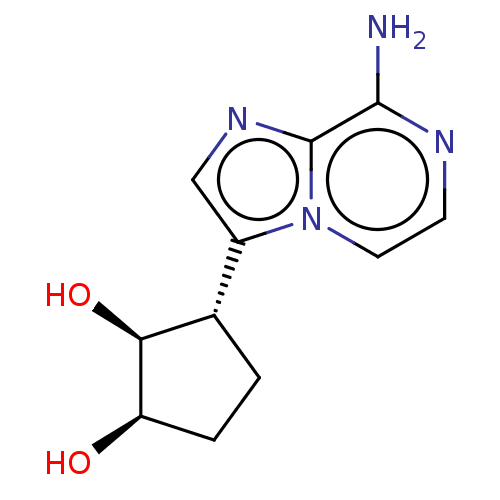
(CHEMBL3290650)Show SMILES Nc1nccn2c(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H14N4O2/c12-10-11-14-5-7(15(11)4-3-13-10)6-1-2-8(16)9(6)17/h3-6,8-9,16-17H,1-2H2,(H2,12,13)/t6-,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018508
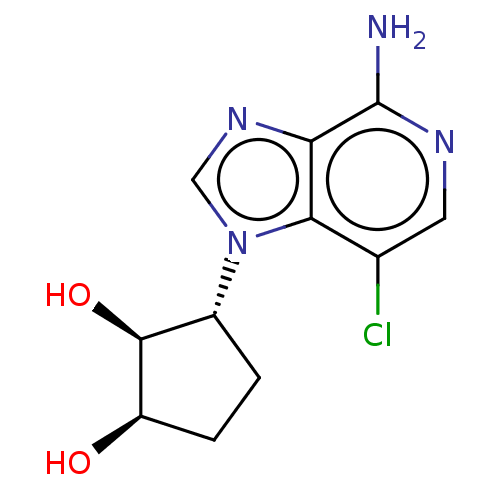
(CHEMBL3290658)Show SMILES Nc1ncc(Cl)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13ClN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018533
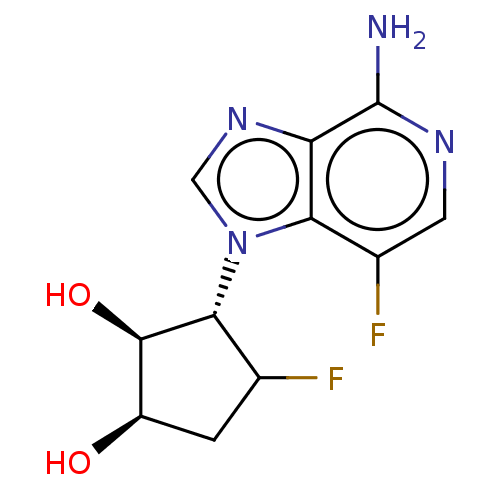
(CHEMBL3290668)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C(F)C[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(18)10(19)9(4)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4?,6-,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018514
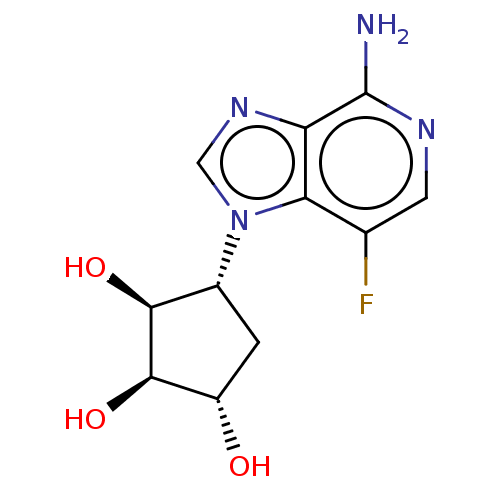
(CHEMBL3290663)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6+,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018508

(CHEMBL3290658)Show SMILES Nc1ncc(Cl)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13ClN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018514

(CHEMBL3290663)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6+,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018529
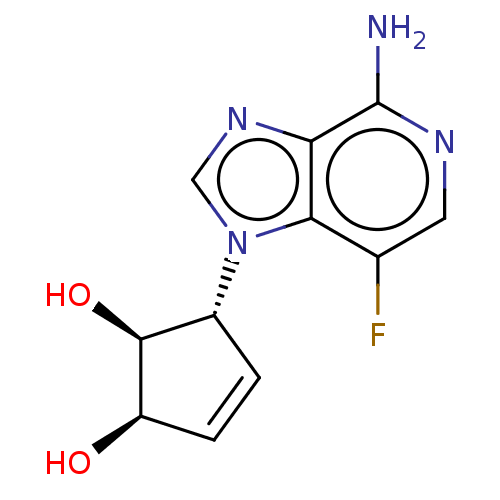
(CHEMBL3290665)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |r,c:14| Show InChI InChI=1S/C11H11FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h1-4,6-7,10,17-18H,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018509
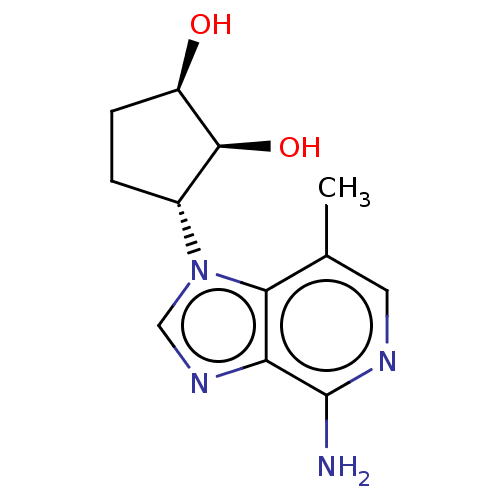
(CHEMBL3290659)Show SMILES Cc1cnc(N)c2ncn([C@@H]3CC[C@@H](O)[C@H]3O)c12 |r| Show InChI InChI=1S/C12H16N4O2/c1-6-4-14-12(13)9-10(6)16(5-15-9)7-2-3-8(17)11(7)18/h4-5,7-8,11,17-18H,2-3H2,1H3,(H2,13,14)/t7-,8-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018507

(CHEMBL3290657)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018512
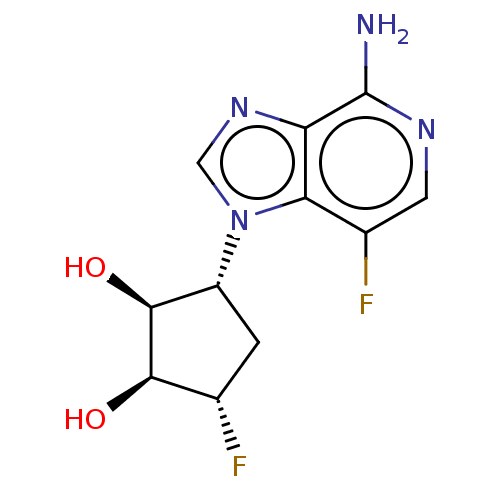
(CHEMBL3290662)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(10(19)9(4)18)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4-,6+,9+,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006218

((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...)Show InChI InChI=1S/C10H13N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h3-6,8,16-17H,1-2H2,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018505
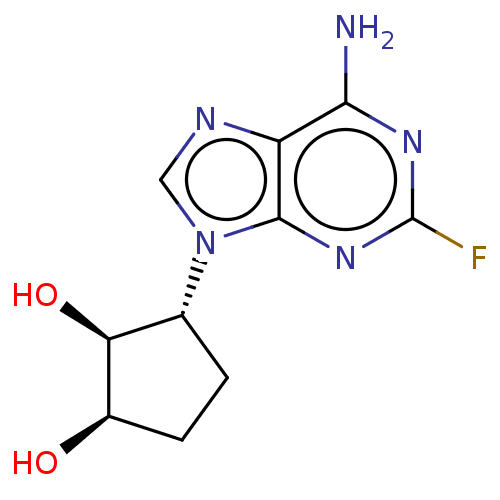
(CHEMBL3290655)Show SMILES Nc1nc(F)nc2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12FN5O2/c11-10-14-8(12)6-9(15-10)16(3-13-6)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,14,15)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018510
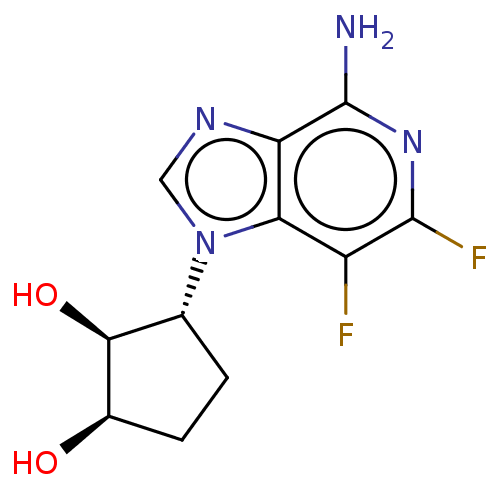
(CHEMBL3290660)Show SMILES Nc1nc(F)c(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-6-8-7(11(14)16-10(6)13)15-3-17(8)4-1-2-5(18)9(4)19/h3-5,9,18-19H,1-2H2,(H2,14,16)/t4-,5-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018500
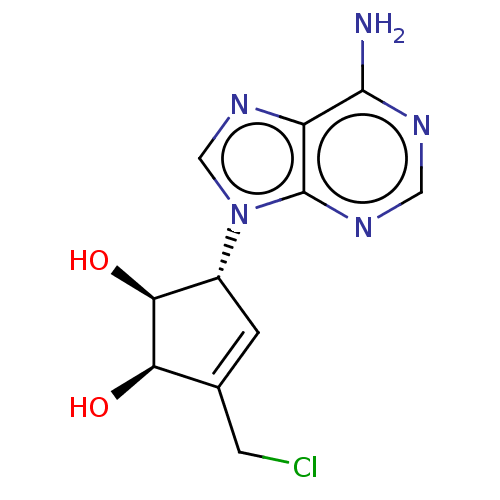
(CHEMBL419393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CCl)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C11H12ClN5O2/c12-2-5-1-6(9(19)8(5)18)17-4-16-7-10(13)14-3-15-11(7)17/h1,3-4,6,8-9,18-19H,2H2,(H2,13,14,15)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018509

(CHEMBL3290659)Show SMILES Cc1cnc(N)c2ncn([C@@H]3CC[C@@H](O)[C@H]3O)c12 |r| Show InChI InChI=1S/C12H16N4O2/c1-6-4-14-12(13)9-10(6)16(5-15-9)7-2-3-8(17)11(7)18/h4-5,7-8,11,17-18H,2-3H2,1H3,(H2,13,14)/t7-,8-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018501

(CHEMBL3290650)Show SMILES Nc1nccn2c(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H14N4O2/c12-10-11-14-5-7(15(11)4-3-13-10)6-1-2-8(16)9(6)17/h3-6,8-9,16-17H,1-2H2,(H2,12,13)/t6-,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018512

(CHEMBL3290662)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(10(19)9(4)18)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4-,6+,9+,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50088426

((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H15N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h3-6,8-9,17-19H,1-2H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018533

(CHEMBL3290668)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C(F)C[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(18)10(19)9(4)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4?,6-,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018511
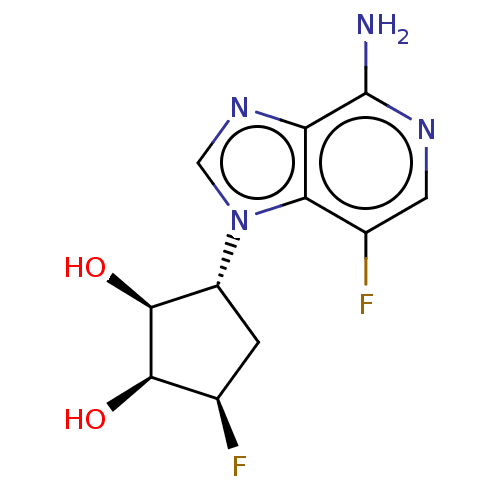
(CHEMBL3290661)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@@H](F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(10(19)9(4)18)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4-,6-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 672 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006215
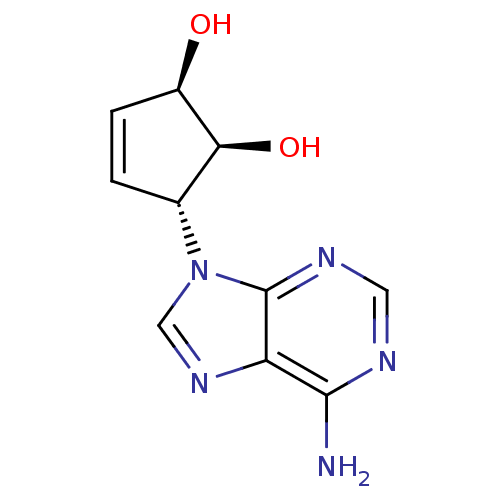
((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C10H11N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h1-6,8,16-17H,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 877 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018513
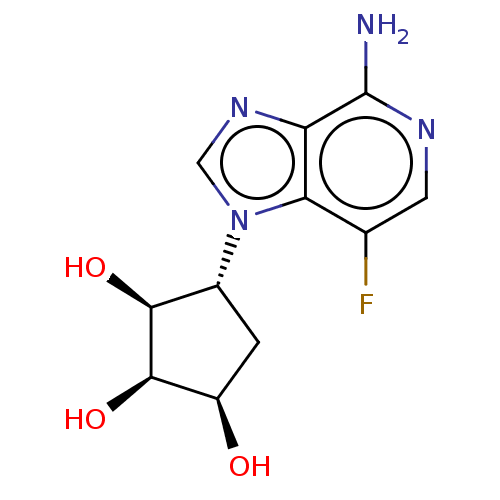
(CHEMBL3290649)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6-,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 878 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018500

(CHEMBL419393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CCl)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C11H12ClN5O2/c12-2-5-1-6(9(19)8(5)18)17-4-16-7-10(13)14-3-15-11(7)17/h1,3-4,6,8-9,18-19H,2H2,(H2,13,14,15)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 939 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018532
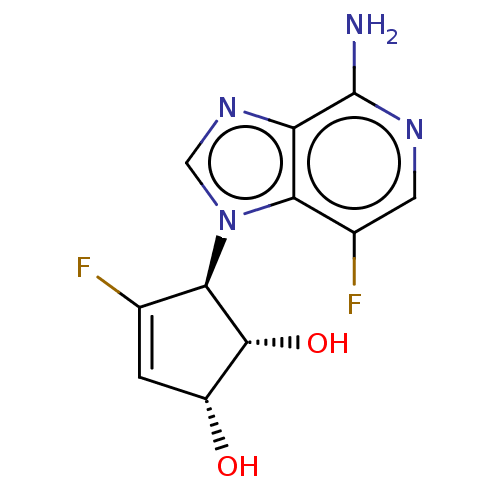
(CHEMBL3290667)Show SMILES Nc1ncc(F)c2n(cnc12)[C@H]1[C@H](O)[C@H](O)C=C1F |r,c:18| Show InChI InChI=1S/C11H10F2N4O2/c12-4-1-6(18)10(19)9(4)17-3-16-7-8(17)5(13)2-15-11(7)14/h1-3,6,9-10,18-19H,(H2,14,15)/t6-,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 976 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018528
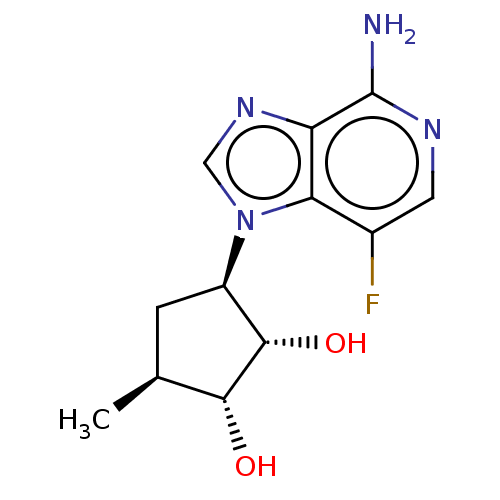
(CHEMBL3290664)Show SMILES C[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncc(F)c12 |r| Show InChI InChI=1S/C12H15FN4O2/c1-5-2-7(11(19)10(5)18)17-4-16-8-9(17)6(13)3-15-12(8)14/h3-5,7,10-11,18-19H,2H2,1H3,(H2,14,15)/t5-,7+,10+,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018530
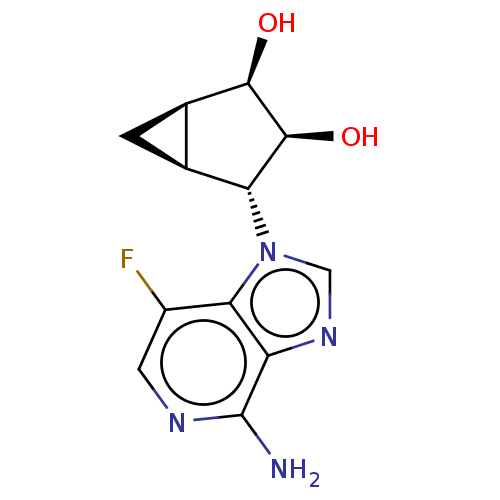
(CHEMBL3290666)Show SMILES [H][C@@]12C[C@]1([H])[C@H]([C@H](O)[C@@H]2O)n1cnc2c(N)ncc(F)c12 |r| Show InChI InChI=1S/C12H13FN4O2/c13-6-2-15-12(14)7-9(6)17(3-16-7)8-4-1-5(4)10(18)11(8)19/h2-5,8,10-11,18-19H,1H2,(H2,14,15)/t4-,5+,8+,10+,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018503
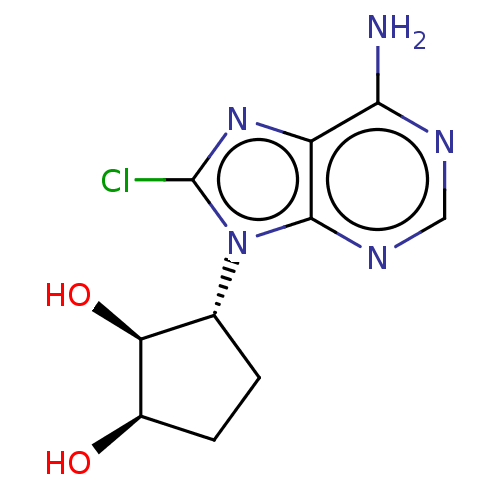
(CHEMBL3290653)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(Cl)nc12 |r| Show InChI InChI=1S/C10H12ClN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018505

(CHEMBL3290655)Show SMILES Nc1nc(F)nc2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12FN5O2/c11-10-14-8(12)6-9(15-10)16(3-13-6)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,14,15)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018510

(CHEMBL3290660)Show SMILES Nc1nc(F)c(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-6-8-7(11(14)16-10(6)13)15-3-17(8)4-1-2-5(18)9(4)19/h3-5,9,18-19H,1-2H2,(H2,14,16)/t4-,5-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006215

((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C10H11N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h1-6,8,16-17H,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018506
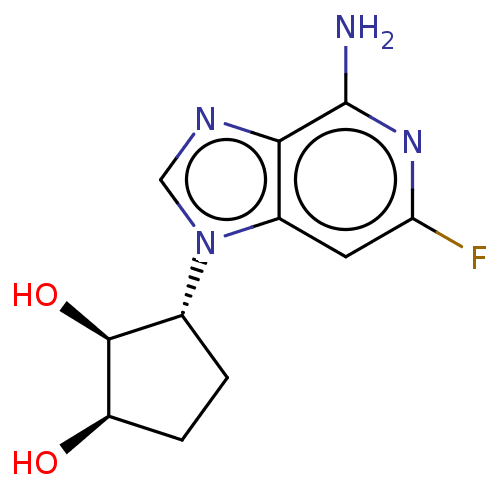
(CHEMBL3290656)Show SMILES Nc1nc(F)cc2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O2/c12-8-3-6-9(11(13)15-8)14-4-16(6)5-1-2-7(17)10(5)18/h3-5,7,10,17-18H,1-2H2,(H2,13,15)/t5-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018529

(CHEMBL3290665)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |r,c:14| Show InChI InChI=1S/C11H11FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h1-4,6-7,10,17-18H,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018530

(CHEMBL3290666)Show SMILES [H][C@@]12C[C@]1([H])[C@H]([C@H](O)[C@@H]2O)n1cnc2c(N)ncc(F)c12 |r| Show InChI InChI=1S/C12H13FN4O2/c13-6-2-15-12(14)7-9(6)17(3-16-7)8-4-1-5(4)10(18)11(8)19/h2-5,8,10-11,18-19H,1H2,(H2,14,15)/t4-,5+,8+,10+,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018504
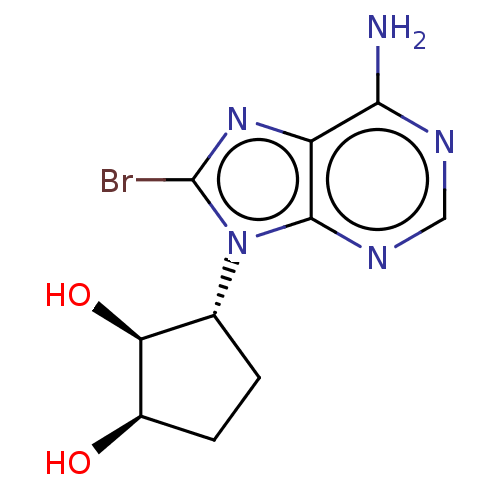
(CHEMBL3290654)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(Br)nc12 |r| Show InChI InChI=1S/C10H12BrN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018503

(CHEMBL3290653)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(Cl)nc12 |r| Show InChI InChI=1S/C10H12ClN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018502
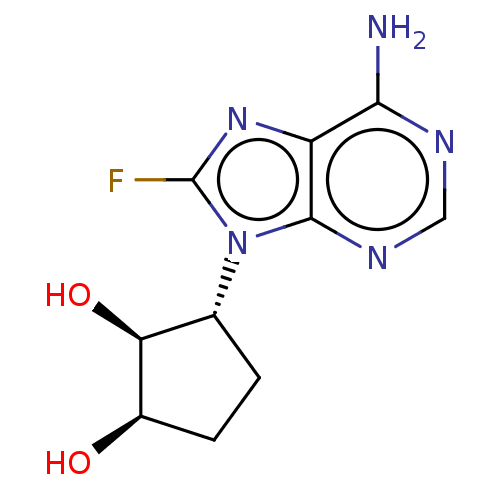
(CHEMBL3290652)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(F)nc12 |r| Show InChI InChI=1S/C10H12FN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018502

(CHEMBL3290652)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(F)nc12 |r| Show InChI InChI=1S/C10H12FN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018511

(CHEMBL3290661)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@@H](F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(10(19)9(4)18)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4-,6-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018513

(CHEMBL3290649)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6-,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018504

(CHEMBL3290654)Show SMILES Nc1ncnc2n([C@@H]3CC[C@@H](O)[C@H]3O)c(Br)nc12 |r| Show InChI InChI=1S/C10H12BrN5O2/c11-10-15-6-8(12)13-3-14-9(6)16(10)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,13,14)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data