

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121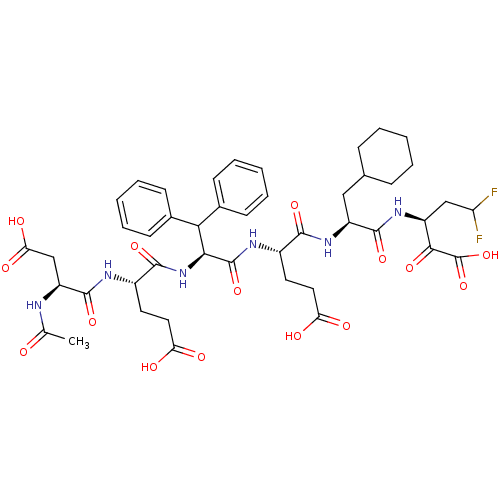 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110153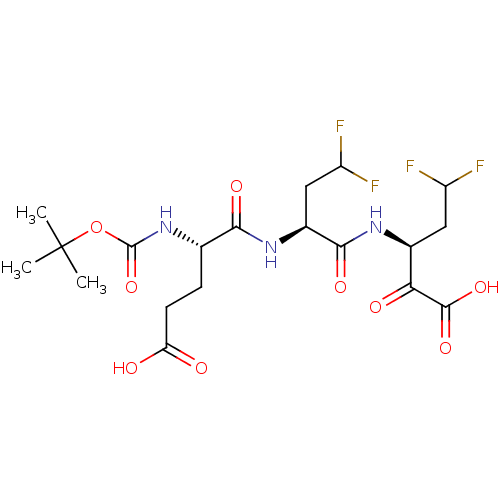 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110146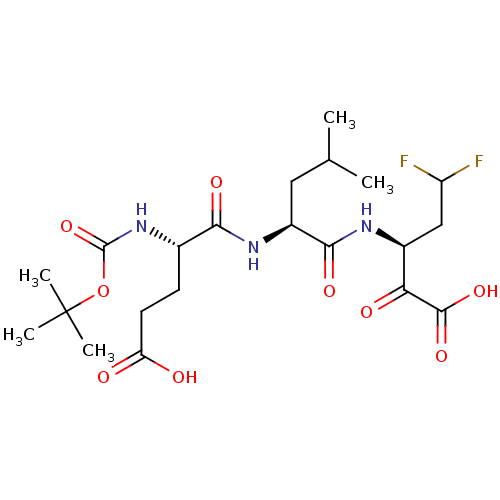 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110145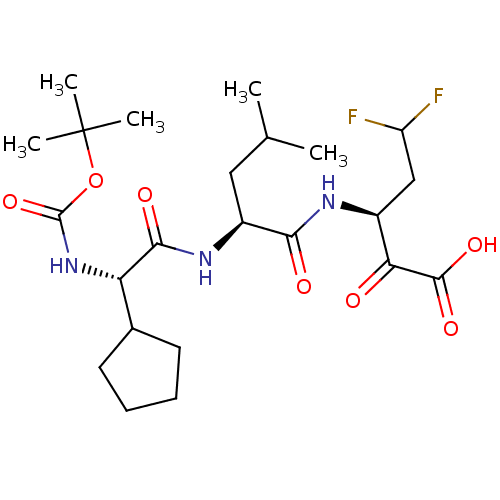 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-2-cyc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110143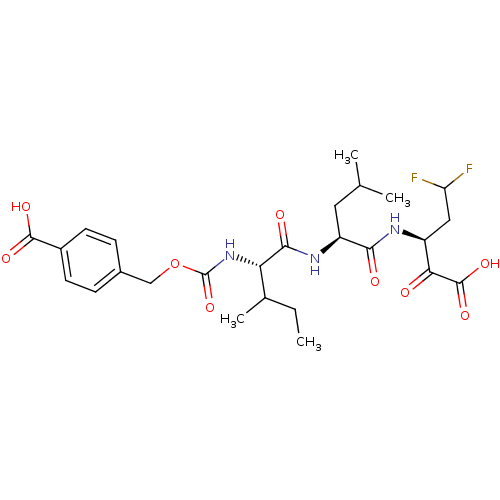 (4-{(S)-1-[(S)-1-((S)-3,3-Difluoro-1-oxalyl-propylc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110134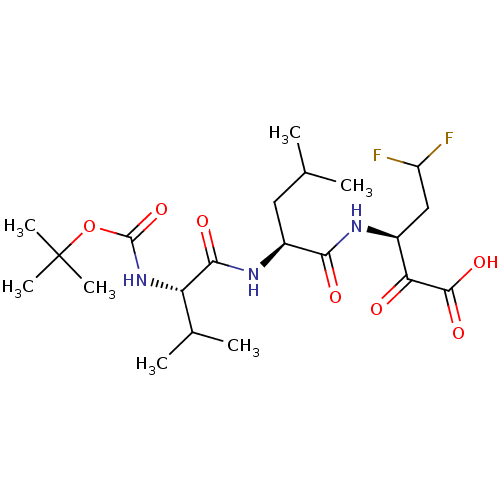 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110130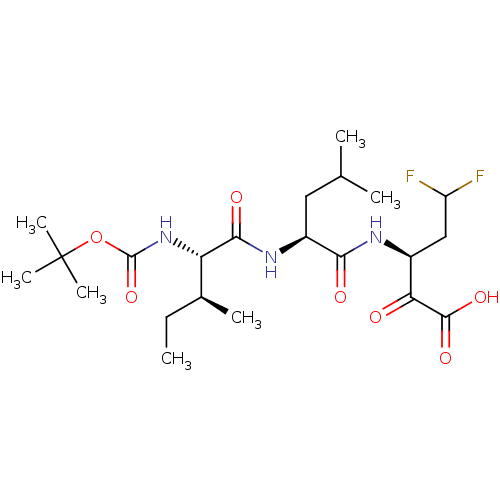 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110156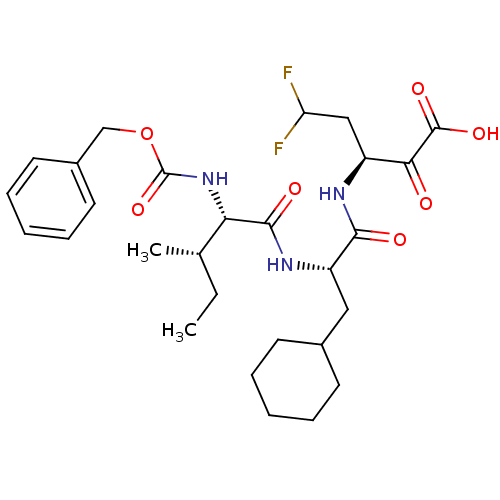 ((S)-3-[(S)-2-((2S,3S)-2-Benzyloxycarbonylamino-3-m...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110148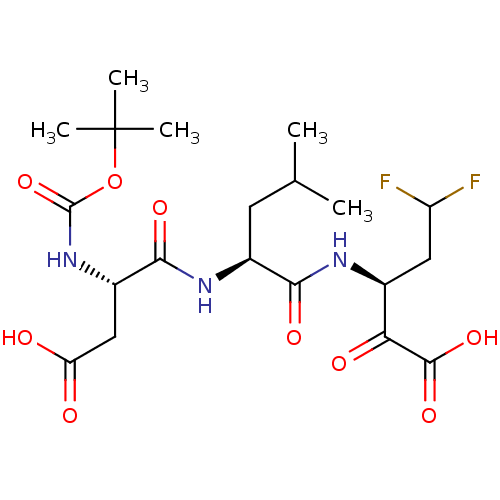 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110152 ((S)-3-[(S)-2-((2S,3S)-2-Benzyloxycarbonylamino-3-m...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110149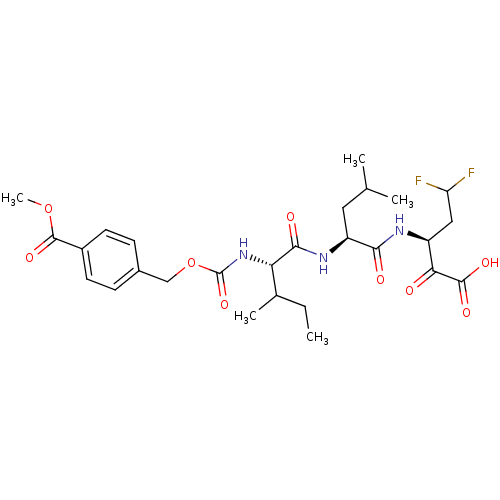 (4-{(S)-1-[(S)-1-((S)-3,3-Difluoro-1-oxalyl-propylc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110129 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110141 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110133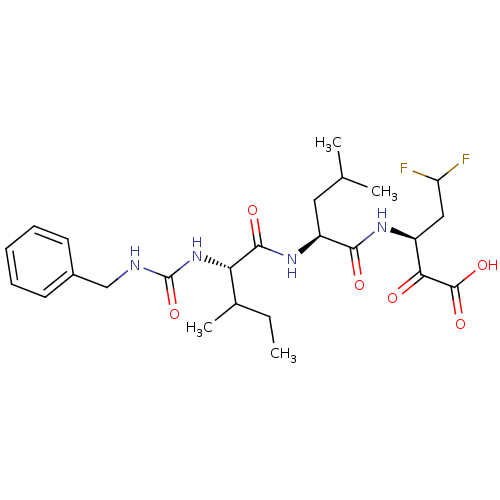 (3-{(1S,2S)-2-[2-(3-Benzyl-ureido)-3-methyl-pentano...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110154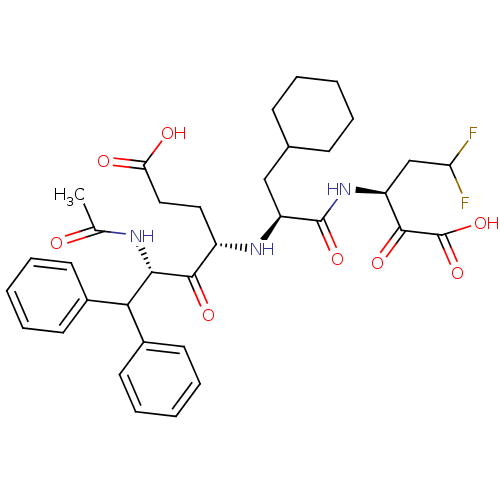 ((4S,6S)-6-Acetylamino-4-[(S)-2-cyclohexyl-1-((S)-3...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110140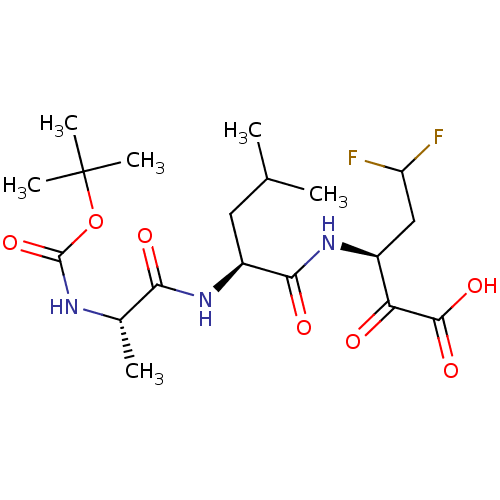 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-propi...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110157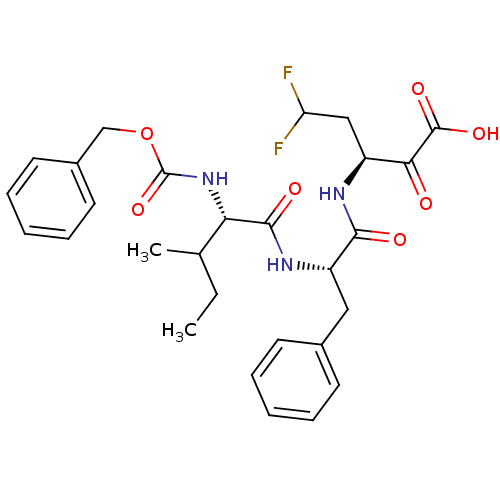 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110131 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110155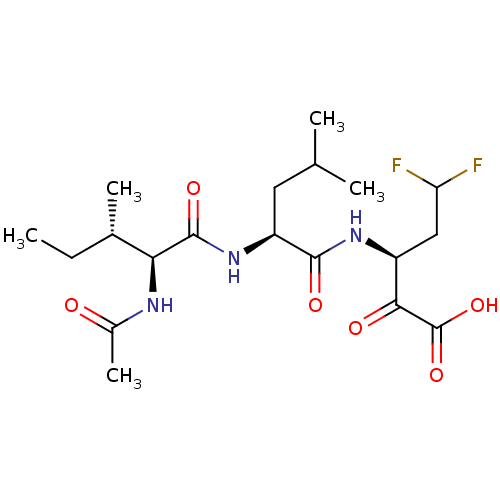 ((S)-3-[(S)-2-((S)-2-Acetylamino-3-methyl-pentanoyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110142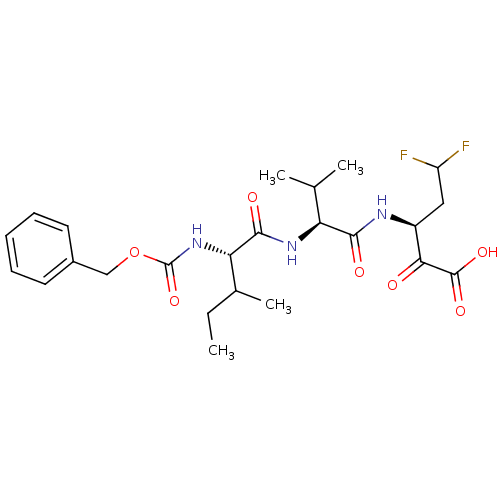 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110151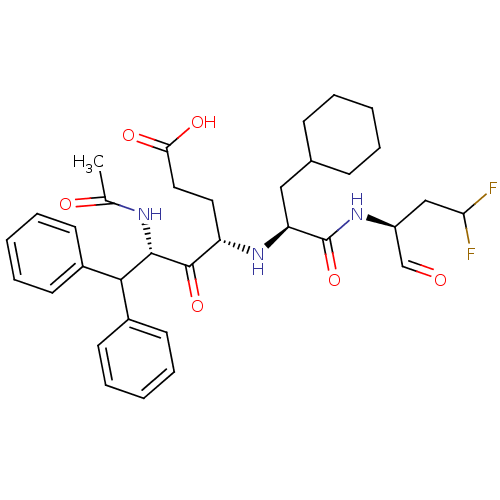 ((4S,6S)-6-Acetylamino-4-[(S)-2-cyclohexyl-1-((S)-3...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110147 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110144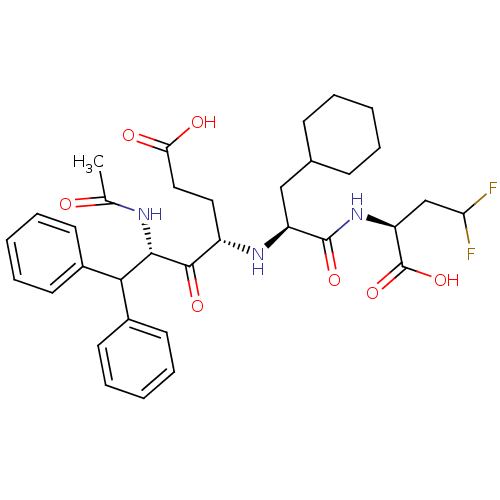 ((4S,6S)-6-Acetylamino-4-[(S)-1-((S)-1-carboxy-3,3-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110136 (CHEMBL2371293 | {(S)-1-[(S)-1-((S)-3,3-Difluoro-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110135 (((S)-1-{(S)-1-[(S)-3,3-Difluoro-1-(1H-tetrazole-5-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110158 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110139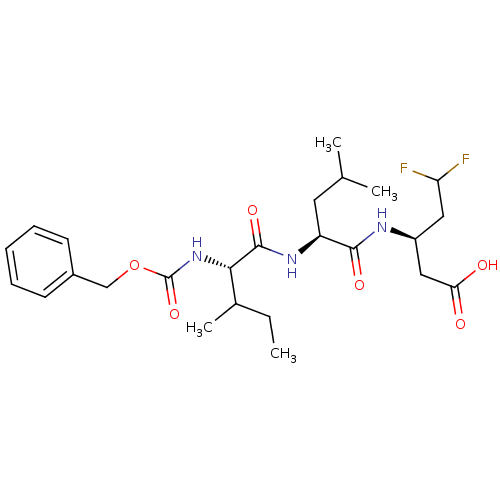 ((R)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110150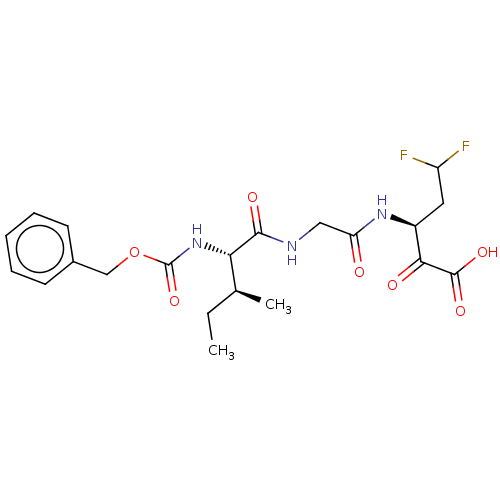 ((S)-3-[2-((S)-2-Benzyloxycarbonylamino-3-methyl-pe...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||