

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069245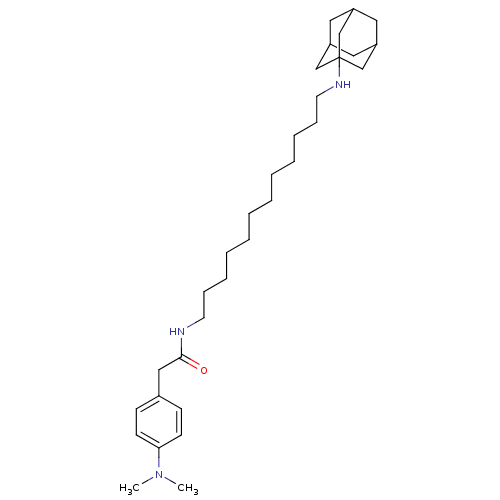 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069248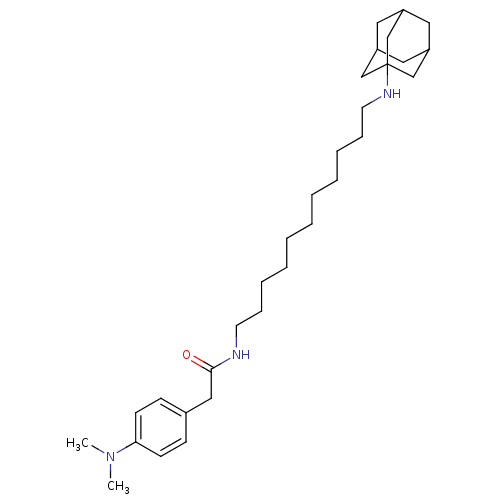 (CHEMBL421974 | N-[11-(Adamantan-1-ylamino)-undecyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069243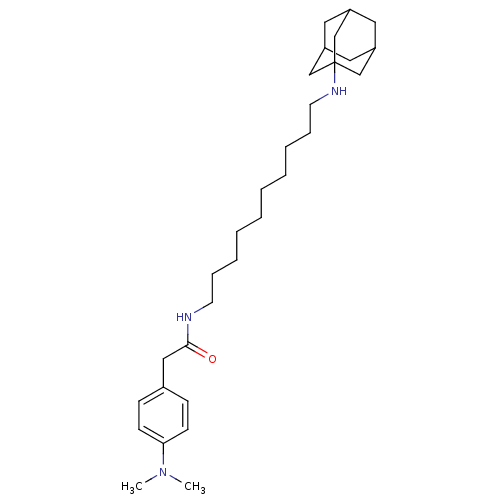 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069247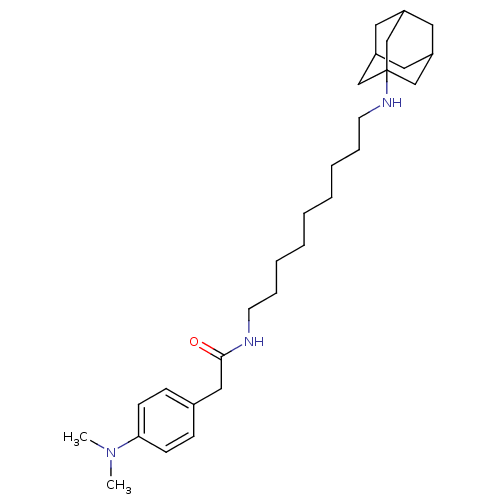 (CHEMBL157429 | N-[9-(Adamantan-1-ylamino)-nonyl]-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069246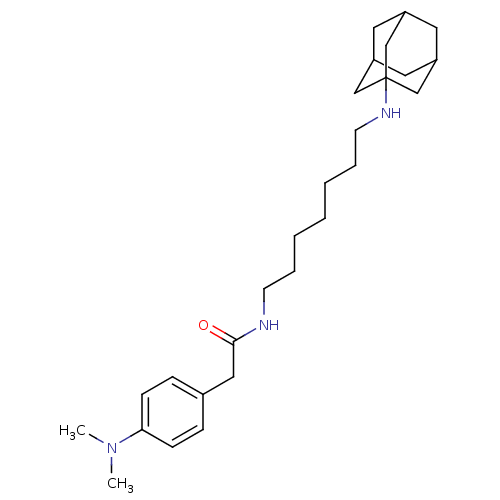 (CHEMBL152361 | N-[7-(Adamantan-1-ylamino)-heptyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069245 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069243 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50069245 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50069243 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069245 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069245 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069243 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069243 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50069243 (CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50069244 (CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50069245 (CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel | Bioorg Med Chem Lett 8: 575-80 (1999) BindingDB Entry DOI: 10.7270/Q2S181N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||