

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin binding to human CCR3 receptors | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098636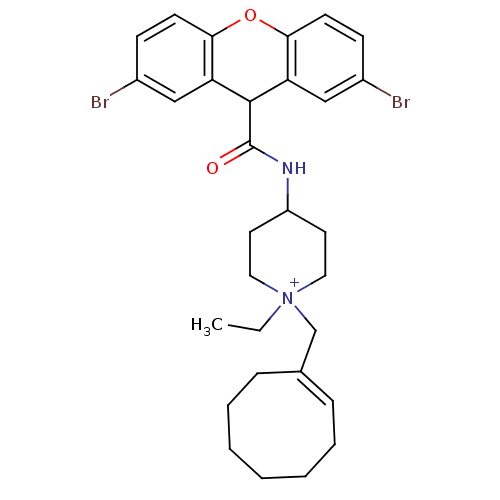 (1-Cyclooct-1-enylmethyl-4-[(2,7-dibromo-9H-xanthen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098641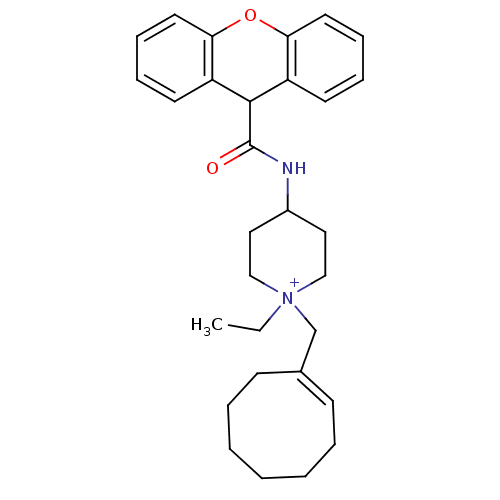 (1-Cyclooct-1-enylmethyl-1-ethyl-4-[(9H-xanthene-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098651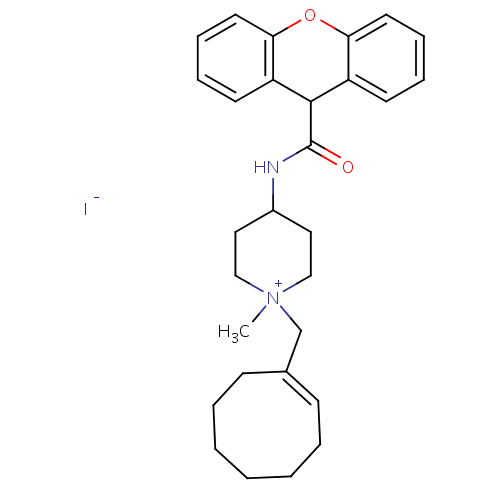 (1-Cyclooct-1-enylmethyl-1-methyl-4-[(9H-xanthene-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098628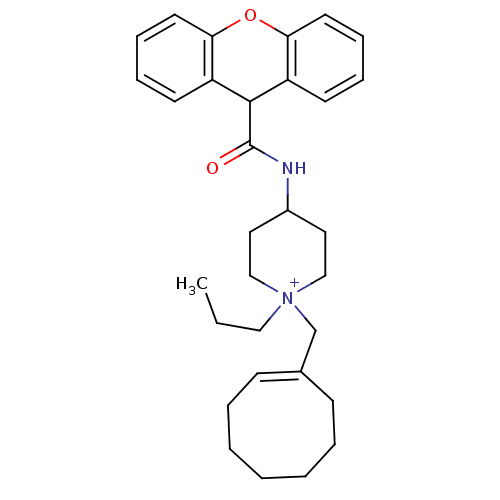 (1-Cyclooct-1-enylmethyl-1-propyl-4-[(9H-xanthene-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098633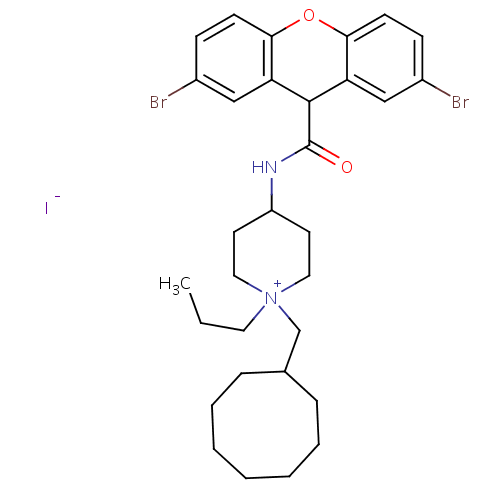 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098648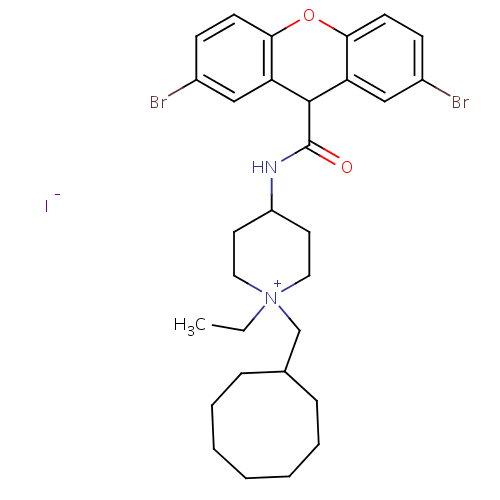 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098637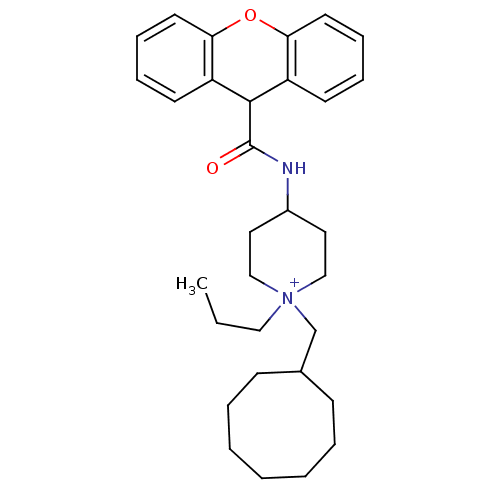 (1-Cyclooctylmethyl-1-propyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098627 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098642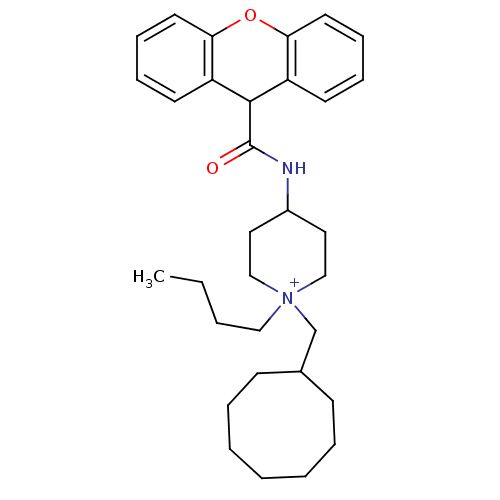 (1-Butyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098626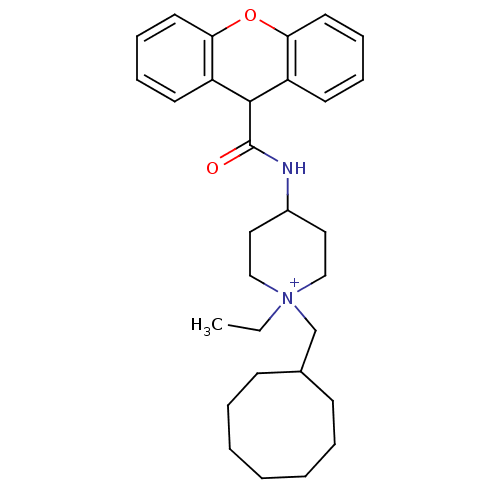 (1-Cyclooctylmethyl-1-ethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against 125 I -MIP-1 alpha binding to mouse CCR1 receptors | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin induced [Ca2+] response in human CCR3 receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098620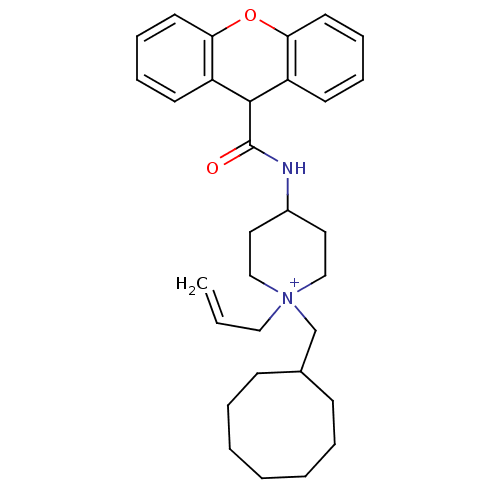 (1-Allyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098636 (1-Cyclooct-1-enylmethyl-4-[(2,7-dibromo-9H-xanthen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098634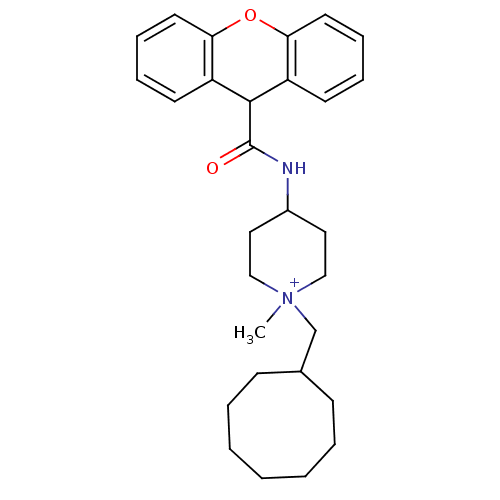 (1-Cyclooctylmethyl-1-methyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098630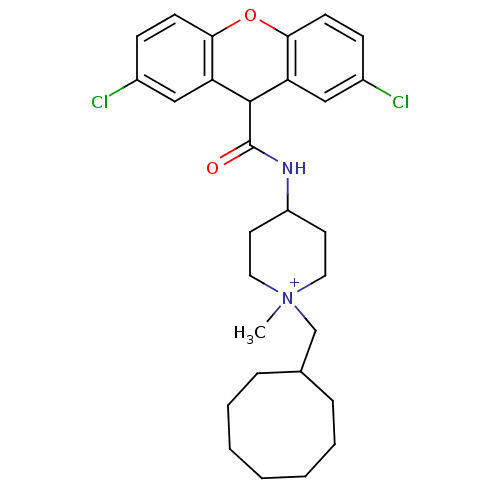 (1-Cyclooctylmethyl-4-[(2,7-dichloro-9H-xanthene-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description inhibitory activity against MIP-1 alpha- induced [Ca2+] response in U937 cells expressing mouse CCR1 receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50027216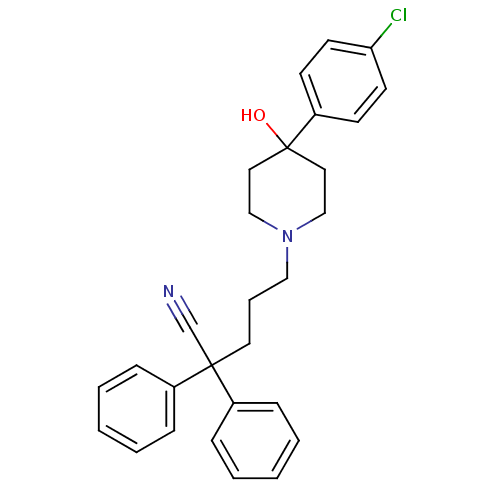 (5-(4-(4-chlorophenyl)-4-hydroxy-piperidin-1-yl)-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098640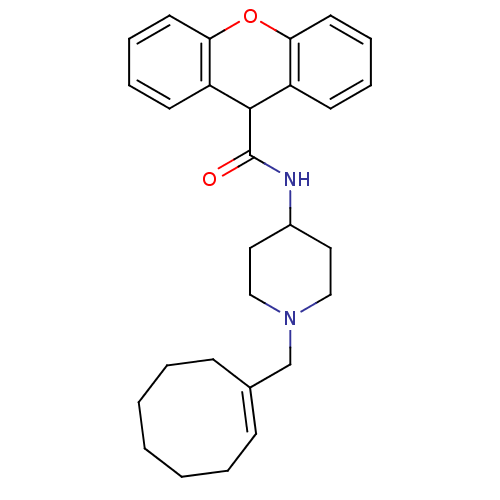 (9H-Xanthene-9-carboxylic acid (1-cyclooct-1-enylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098641 (1-Cyclooct-1-enylmethyl-1-ethyl-4-[(9H-xanthene-9-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098650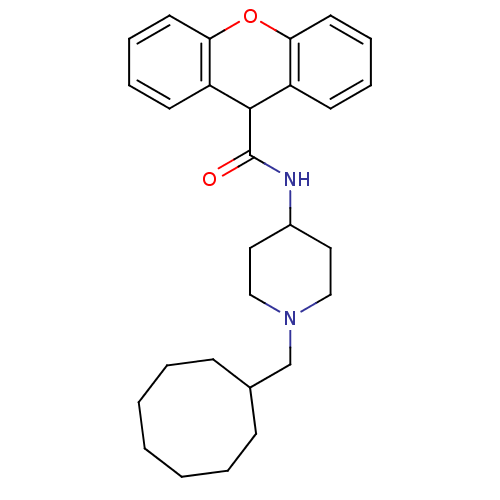 (9H-Xanthene-9-carboxylic acid (1-cyclooctylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098648 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098633 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098635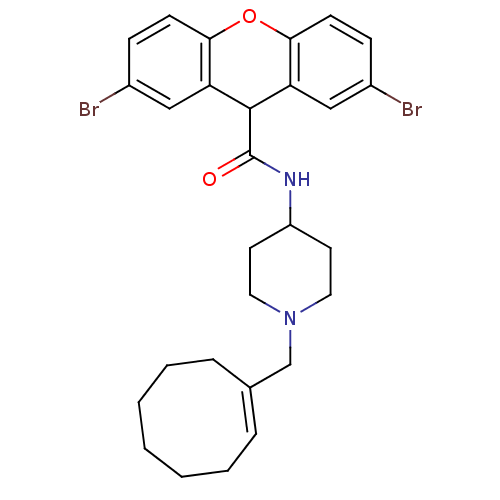 (2,7-Dibromo-9H-xanthene-9-carboxylic acid (1-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098629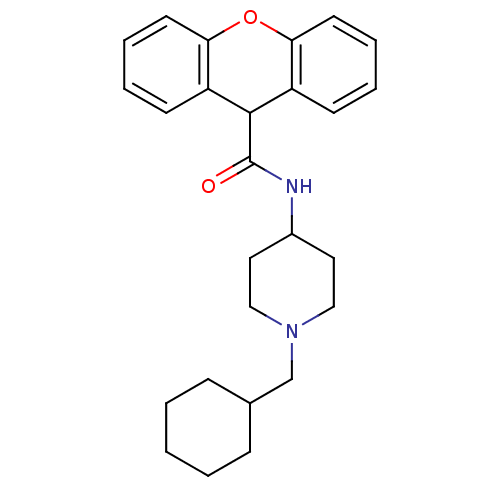 (9H-Xanthene-9-carboxylic acid (1-cyclohexylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098644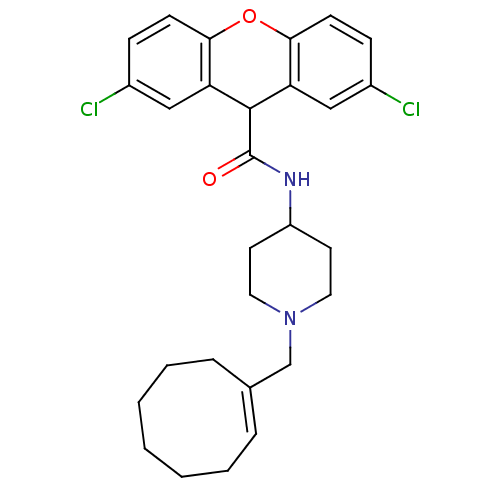 (2,7-Dichloro-9H-xanthene-9-carboxylic acid (1-cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098627 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098637 (1-Cyclooctylmethyl-1-propyl-4-[(9H-xanthene-9-carb...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098628 (1-Cyclooct-1-enylmethyl-1-propyl-4-[(9H-xanthene-9...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098651 (1-Cyclooct-1-enylmethyl-1-methyl-4-[(9H-xanthene-9...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable C-C chemokine receptor type 3 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin induced [Ca2+] response in mouse CCR3 receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098639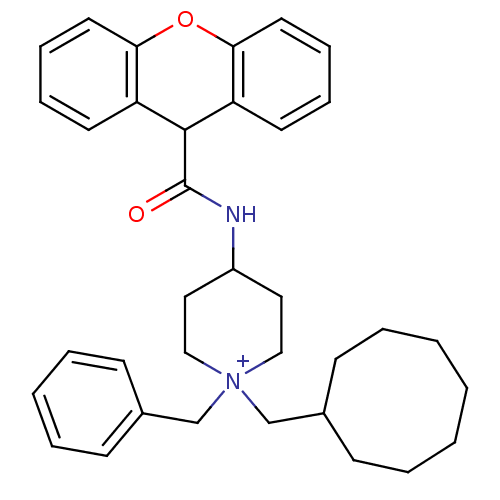 (1-Benzyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098642 (1-Butyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable C-C chemokine receptor type 3 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin alpha binding to mouse CCR3 receptors | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098625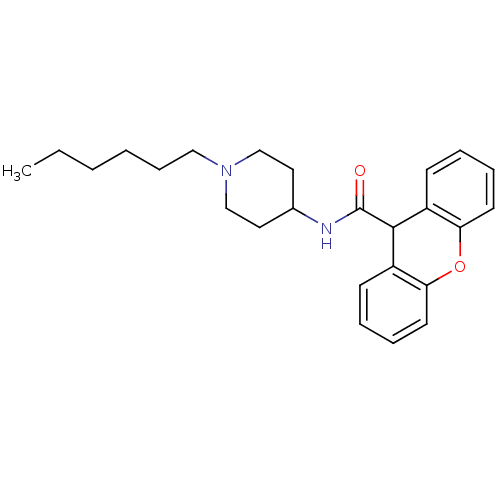 (9H-Xanthene-9-carboxylic acid (1-hexyl-piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098640 (9H-Xanthene-9-carboxylic acid (1-cyclooct-1-enylme...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098626 (1-Cyclooctylmethyl-1-ethyl-4-[(9H-xanthene-9-carbo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098620 (1-Allyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098630 (1-Cyclooctylmethyl-4-[(2,7-dichloro-9H-xanthene-9-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098622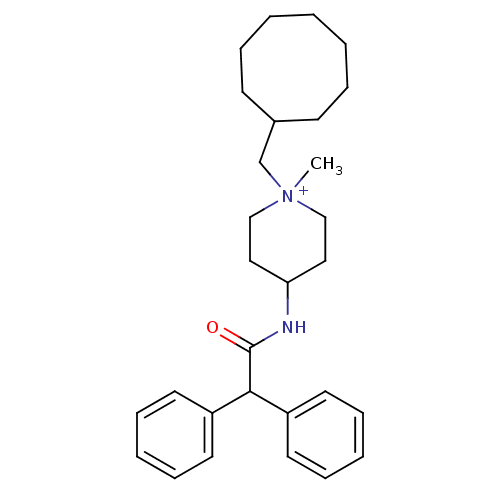 (1-Cyclooctylmethyl-4-diphenylacetylamino-1-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098647 (9H-Xanthene-9-carboxylic acid (1-cyclodecylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of RANTES binding to C-C chemokine receptor type 5 | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity in human CCR2b receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098645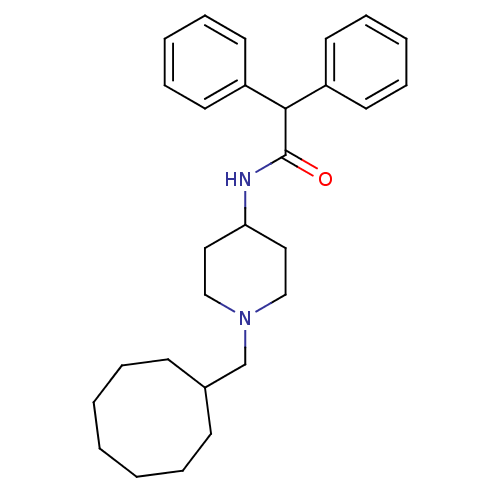 (CHEMBL286355 | N-(1-Cyclooctylmethyl-piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098639 (1-Benzyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carb...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098638 (CHEMBL33159 | N-(1-Cyclooctylmethyl-piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |