

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123880 ((4-Chloro-phenyl)-(2-chloro-pyridine-4-carbonyl)-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Activity of selected HIV-1 N-acylthiocarbamates in enzyme assay against Virion-Associated reverse transcriptase | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123871 ((4-Chloro-phenyl)-(furan-2-carbonyl)-thiocarbamic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Activity of selected HIV-1 N-acylthiocarbamates in enzyme assay against Virion-Associated reverse transcriptase | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123875 ((4-Chloro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Activity of selected HIV-1 N-acylthiocarbamates in enzyme assay against Virion-Associated reverse transcriptase | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Activity of selected HIV-1 N-acylthiocarbamates in enzyme assay against Virion-Associated reverse transcriptase | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Activity of selected HIV-1 N-acylthiocarbamates in enzyme assay against Virion-Associated reverse transcriptase | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123872 ((2-Phenoxy-acetyl)-phenyl-thiocarbamic acid O-(1-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123870 (Benzoyl-phenyl-thiocarbamic acid O-[2-(1,3-dioxo-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123917 ((4-Fluoro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123881 ((4-Ethyl-phenyl)-(furan-2-carbonyl)-thiocarbamic a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123890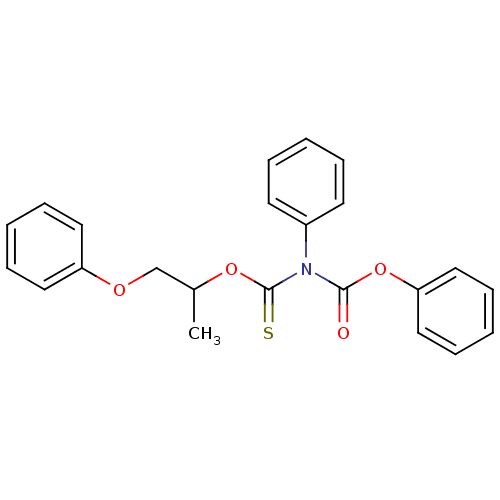 (CHEMBL166333 | O-(1-methyl-2-phenoxyethyl) O-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123895 (Benzoyl-phenyl-thiocarbamic acid O-phenethyl ester...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123916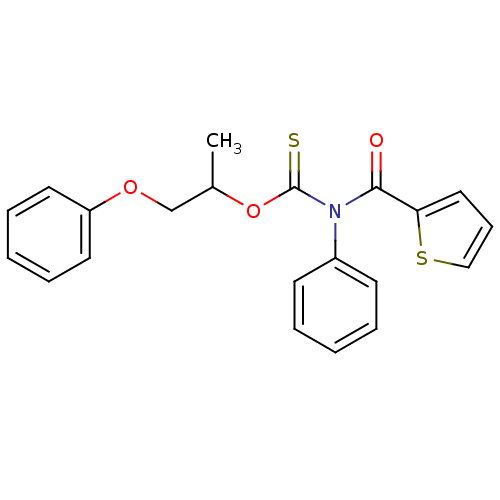 (CHEMBL349827 | Phenyl-(thiophene-2-carbonyl)-thioc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123900 ((Naphthalene-2-carbonyl)-phenyl-thiocarbamic acid ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123921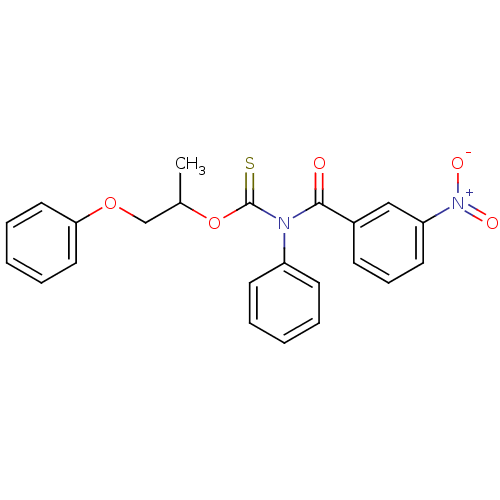 ((3-Nitro-benzoyl)-phenyl-thiocarbamic acid O-(1-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123886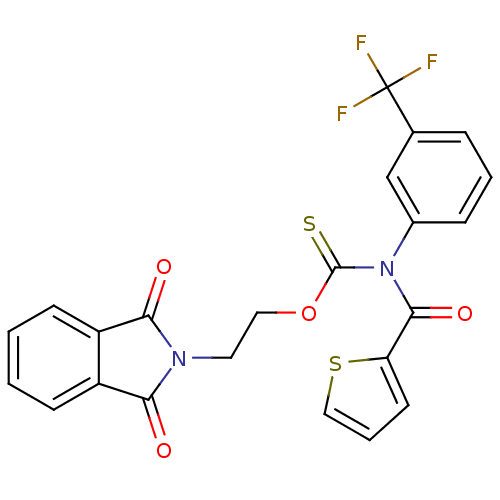 ((Thiophene-2-carbonyl)-(3-trifluoromethyl-phenyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123888 ((2-Ethyl-phenyl)-(thiophene-2-carbonyl)-thiocarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123906 ((3-Chloro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123877 (Benzoyl-phenyl-thiocarbamic acid O-furan-2-ylmethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.83E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123868 ((4-Methyl-benzoyl)-phenyl-thiocarbamic acid O-(2-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123924 ((3-Acetyl-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123909 ((3-Fluoro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123883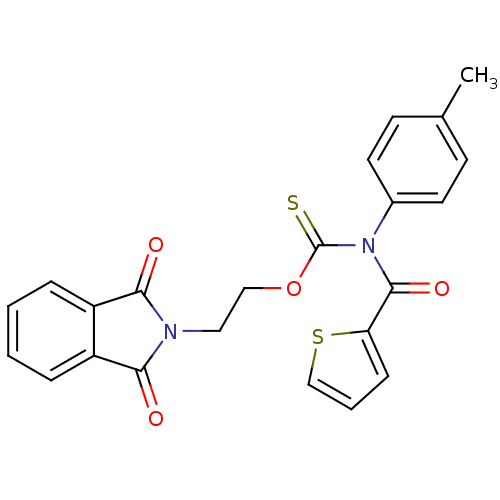 ((Thiophene-2-carbonyl)-p-tolyl-thiocarbamic acid O...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123889 (CHEMBL164697 | Phenyl-(tetrahydro-furan-2-carbonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123905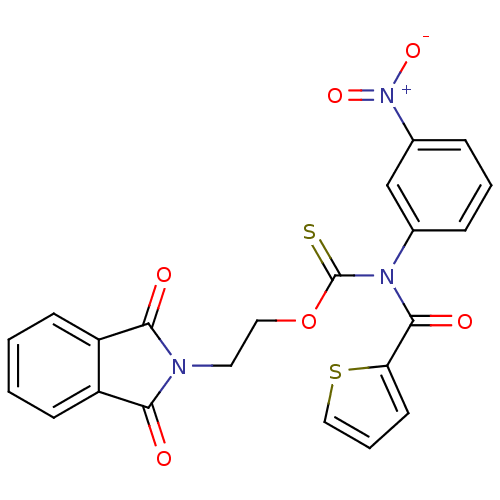 ((3-Nitro-phenyl)-(thiophene-2-carbonyl)-thiocarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123925 ((Thiophene-2-carbonyl)-m-tolyl-thiocarbamic acid O...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123914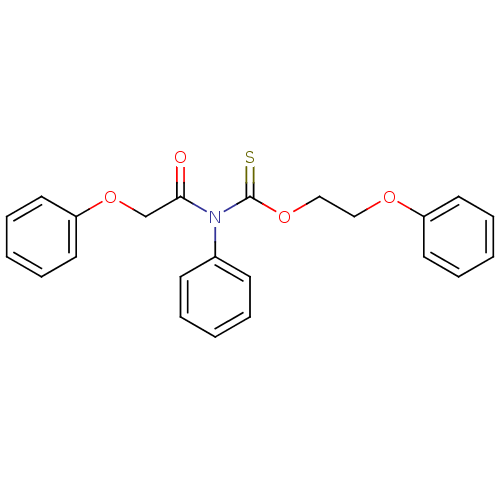 ((2-Phenoxy-acetyl)-phenyl-thiocarbamic acid O-(2-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123923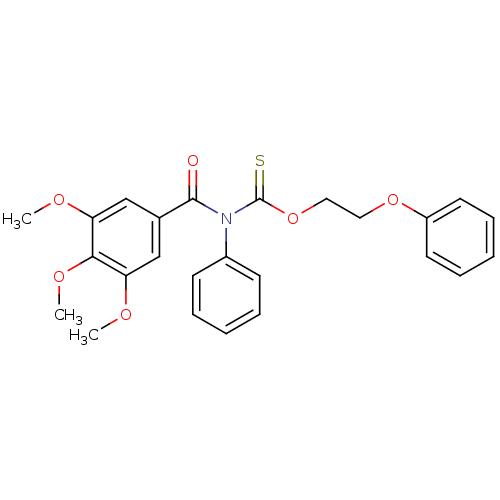 (CHEMBL164503 | Phenyl-(3,4,5-trimethoxy-benzoyl)-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123878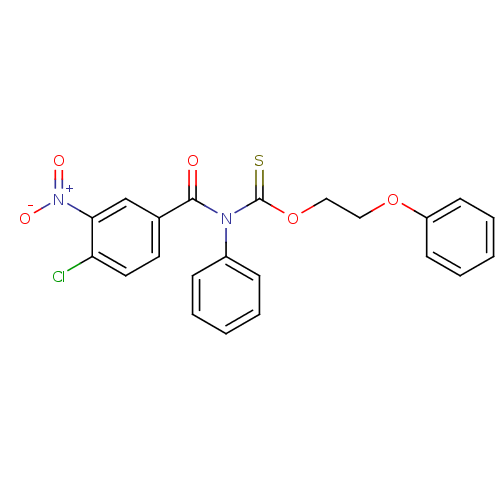 ((4-Chloro-3-nitro-benzoyl)-phenyl-thiocarbamic aci...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123875 ((4-Chloro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123892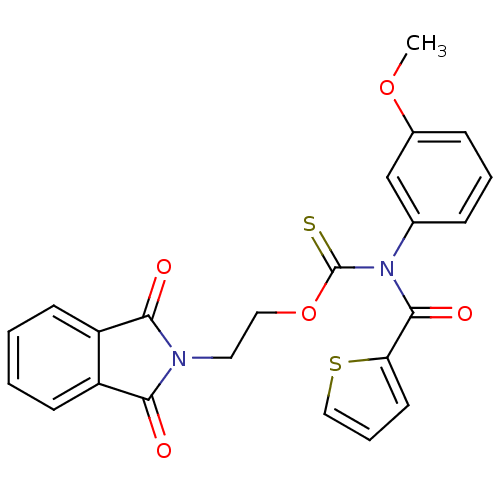 ((3-Methoxy-phenyl)-(thiophene-2-carbonyl)-thiocarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123893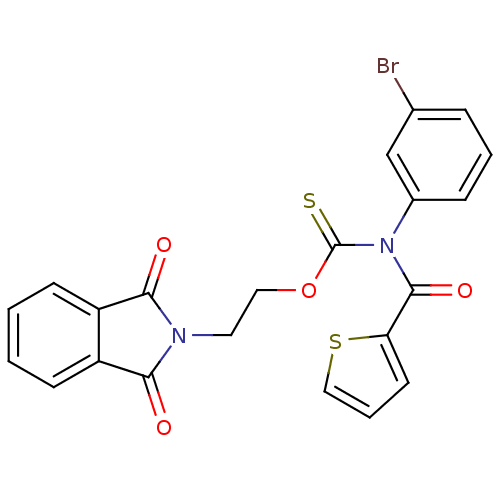 ((3-Bromo-phenyl)-(thiophene-2-carbonyl)-thiocarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123918 ((4-Chloro-benzoyl)-phenyl-thiocarbamic acid O-(2-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123896 ((4-Chloro-benzoyl)-(4-chloro-phenyl)-thiocarbamic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123904 ((3,5-Dichloro-benzoyl)-phenyl-thiocarbamic acid O-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123867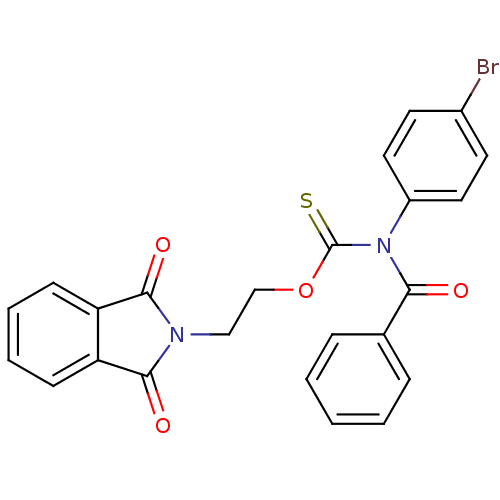 (Benzoyl-(4-bromo-phenyl)-thiocarbamic acid O-[2-(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123879 (Benzoyl-phenyl-thiocarbamic acid O-(2-phenylsulfan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >4.40E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123885 (Benzoyl-(4-fluoro-phenyl)-thiocarbamic acid O-(2-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123912 ((4-Nitro-phenyl)-(thiophene-2-carbonyl)-thiocarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123897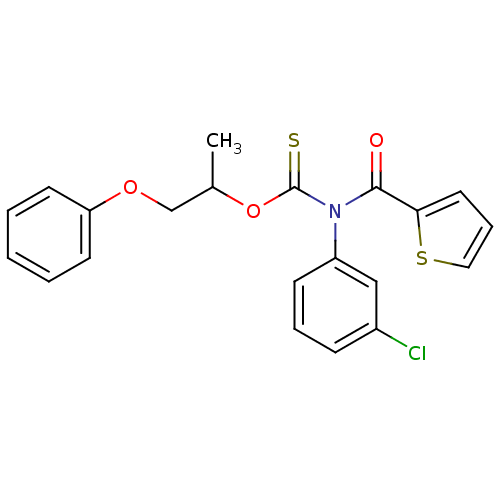 ((3-Chloro-phenyl)-(thiophene-2-carbonyl)-thiocarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123884 ((Furan-2-carbonyl)-phenyl-thiocarbamic acid O-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123894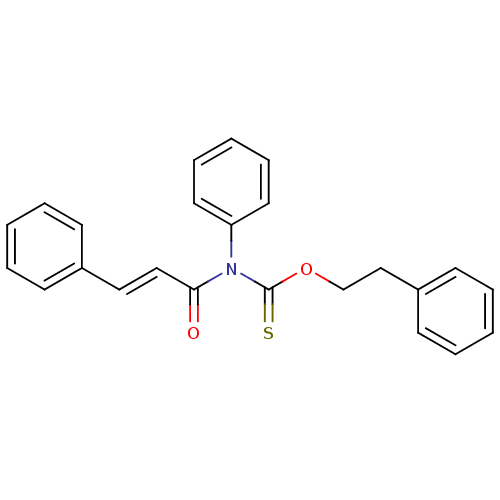 (CHEMBL165288 | Phenyl-(3-phenyl-acryloyl)-thiocarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123898 ((2,4-Dichloro-benzoyl)-phenyl-thiocarbamic acid O-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123901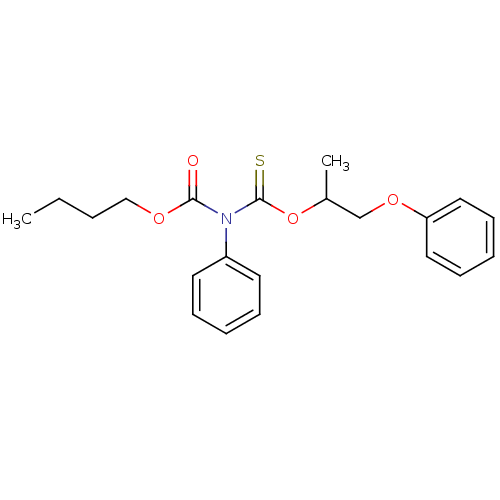 (CHEMBL351212 | O-butyl O-(1-methyl-2-phenoxyethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123880 ((4-Chloro-phenyl)-(2-chloro-pyridine-4-carbonyl)-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123882 (Benzoyl-(4-chloro-phenyl)-thiocarbamic acid O-[2-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123903 (CHEMBL168044 | O-(1-methyl-2-phenoxyethyl) O-propy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123871 ((4-Chloro-phenyl)-(furan-2-carbonyl)-thiocarbamic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123911 ((4-Chloro-benzoyl)-phenyl-thiocarbamic acid O-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50123887 (Benzoyl-cyclohexyl-thiocarbamic acid O-(2-phenoxy-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a |
Università di Genova Curated by ChEMBL | Assay Description Concentration required to achieve 50% protection of MT-4 cell from the HIV-1 induced cytopathogenicity was determined by the MTT method | J Med Chem 46: 768-81 (2003) Article DOI: 10.1021/jm0209984 BindingDB Entry DOI: 10.7270/Q2BC3XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |