

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9235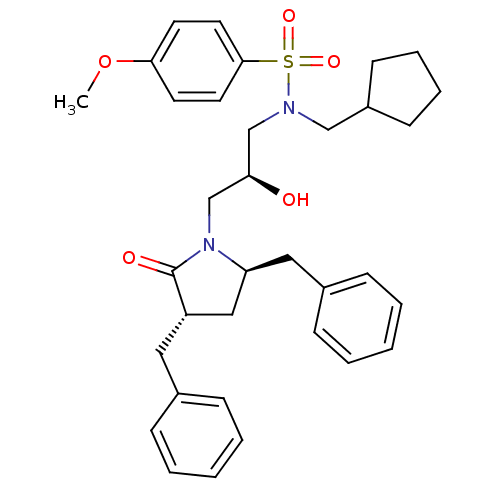 ((2S)-N-(cyclopentylmethyl)-3-[(3S,5R)-3,5-dibenzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073368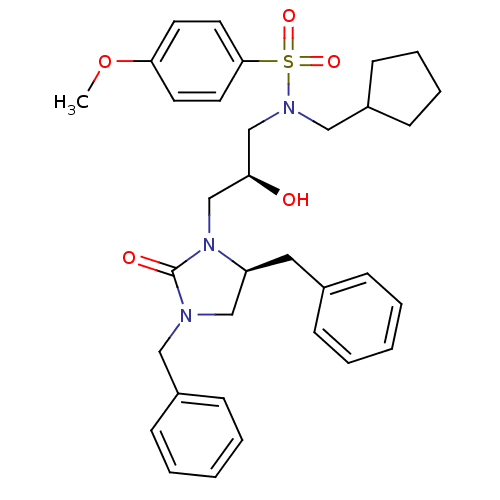 (CHEMBL119490 | N-Cyclopentylmethyl-N-[(S)-3-((S)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073353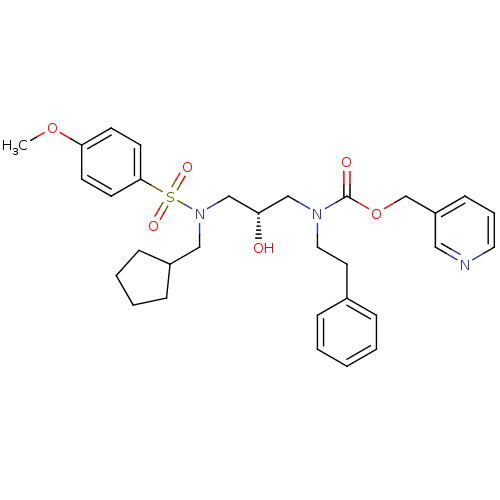 (CHEMBL332611 | {(S)-3-[Cyclopentylmethyl-(4-methox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073369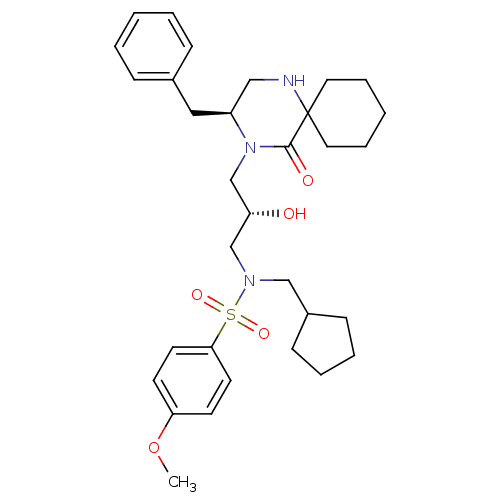 (CHEMBL433187 | N-[(S)-3-((S)-3-Benzyl-5-oxo-1,4-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM13722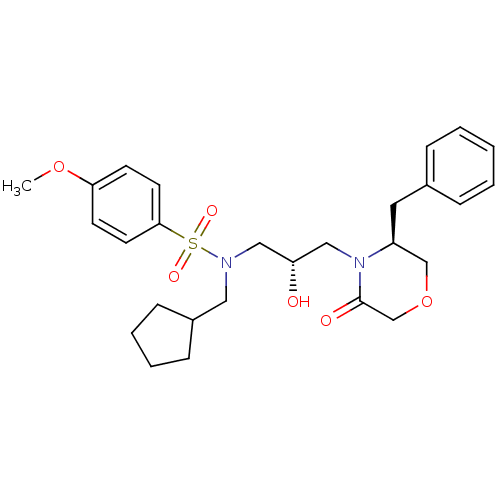 ((2S)-3-[(3S)-3-benzyl-5-oxomorpholin-4-yl]-N-(cycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073367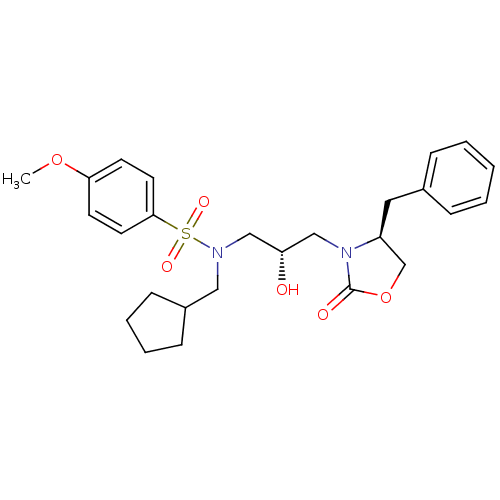 (CHEMBL420158 | N-[(S)-3-((S)-4-Benzyl-2-oxo-oxazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073370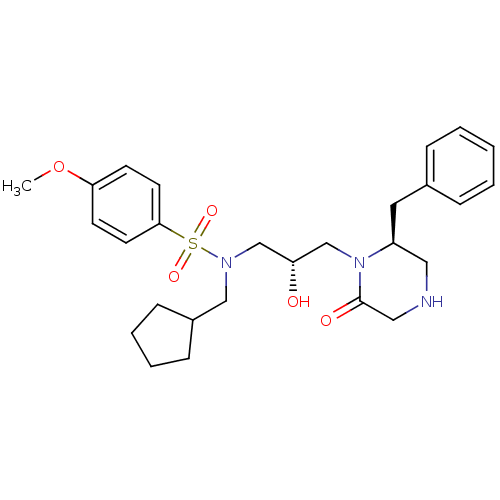 (CHEMBL331441 | N-[(S)-3-((S)-2-Benzyl-6-oxo-pipera...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Compound was assayed for inhibition against HIV protease activity | Bioorg Med Chem Lett 8: 3637-42 (1999) BindingDB Entry DOI: 10.7270/Q2GM86FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||