

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230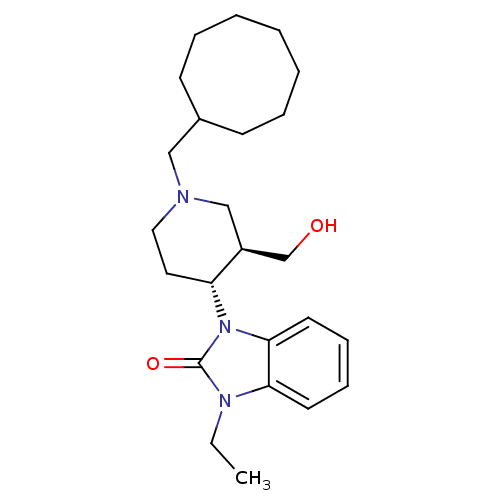 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083227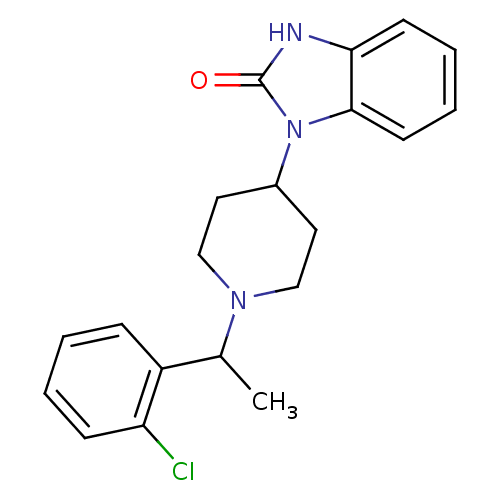 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232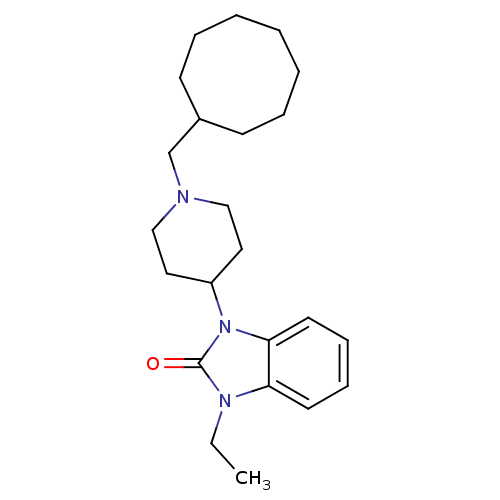 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]U-69593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137562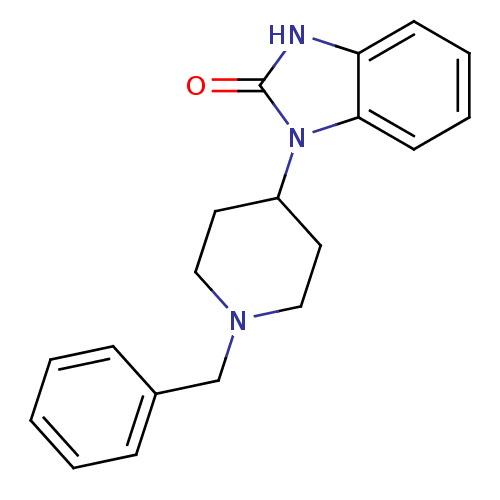 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]U-69593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-diprenorphine binding to human Opioid receptor mu 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-diprenorphine binding to human Opioid receptor mu 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor delta 1 by displacing radioligand [3H][D-Ala2,D-Leu5]enk... | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity against nociceptin produced [35S]GTP-gamma-S, binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to human Opioid receptor delta 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor delta 1 by displacing radioligand [3H][D-Ala2,D-Leu5]enk... | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor delta 1 by displacing radioligand [3H][D-Ala2,D-Leu5]enk... | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to human Opioid receptor delta 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor delta 1 by displacing radioligand [3H][D-Ala2,D-Leu5]enk... | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity against nociceptin produced [35S]GTP-gamma-S, binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound against nociceptin produced GTPgammaS binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083229 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound against nociceptin produced GTPgammaS binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells. | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound against nociceptin produced GTPgammaS binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity of the compound against nociceptin produced GTPgammaS binding to Opioid receptor like 1 expressed in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||