 Found 109 hits Enz. Inhib. hit(s) with all data for entry = 50027480
Found 109 hits Enz. Inhib. hit(s) with all data for entry = 50027480 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275943
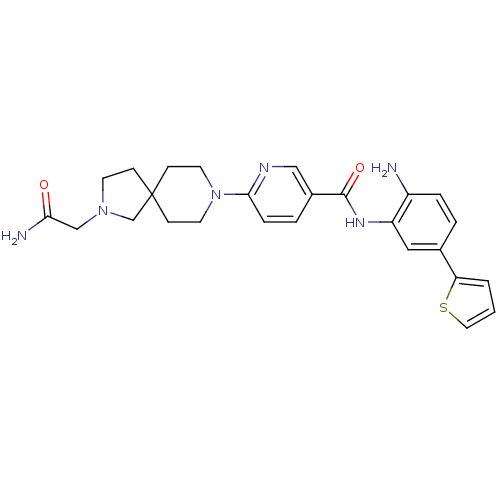
(6-(2-(2-amino-2-oxoethyl)-2,8-diazaspiro[4.5]decan...)Show SMILES NC(=O)CN1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H30N6O2S/c27-20-5-3-18(22-2-1-13-35-22)14-21(20)30-25(34)19-4-6-24(29-15-19)32-11-8-26(9-12-32)7-10-31(17-26)16-23(28)33/h1-6,13-15H,7-12,16-17,27H2,(H2,28,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275941
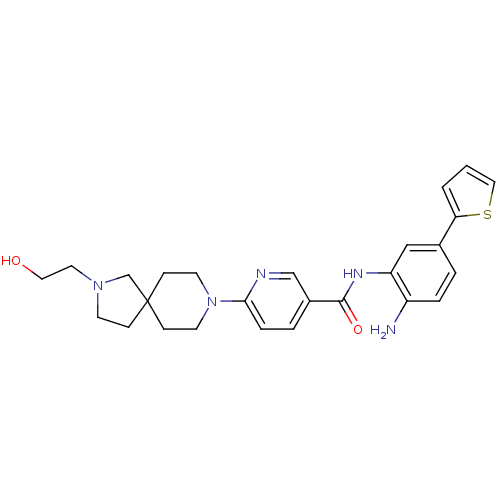
(CHEMBL513250 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCN(CCO)C2)CC1)-c1cccs1 Show InChI InChI=1S/C26H31N5O2S/c27-21-5-3-19(23-2-1-15-34-23)16-22(21)29-25(33)20-4-6-24(28-17-20)31-11-8-26(9-12-31)7-10-30(18-26)13-14-32/h1-6,15-17,32H,7-14,18,27H2,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275886
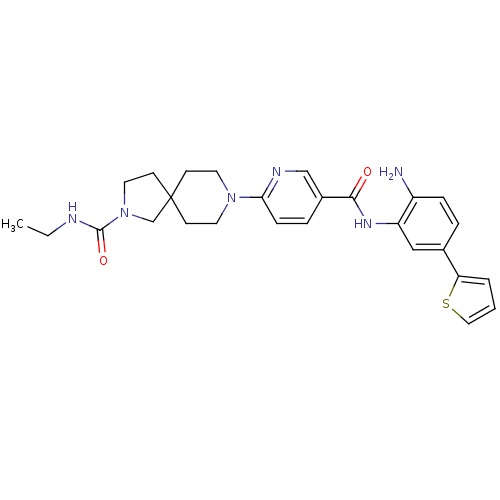
(8-(5-(2-amino-5-(thiophen-2-yl)phenylcarbamoyl)pyr...)Show SMILES CCNC(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C27H32N6O2S/c1-2-29-26(35)33-14-11-27(18-33)9-12-32(13-10-27)24-8-6-20(17-30-24)25(34)31-22-16-19(5-7-21(22)28)23-4-3-15-36-23/h3-8,15-17H,2,9-14,18,28H2,1H3,(H,29,35)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275769
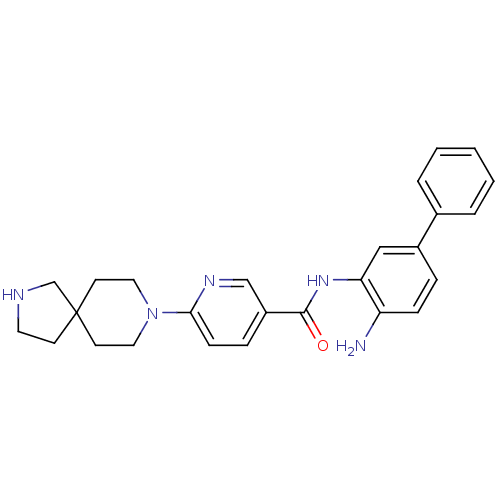
(CHEMBL471824 | N-(4-aminobiphenyl-3-yl)-6-(2,8-dia...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1ccccc1 Show InChI InChI=1S/C26H29N5O/c27-22-8-6-20(19-4-2-1-3-5-19)16-23(22)30-25(32)21-7-9-24(29-17-21)31-14-11-26(12-15-31)10-13-28-18-26/h1-9,16-17,28H,10-15,18,27H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275888
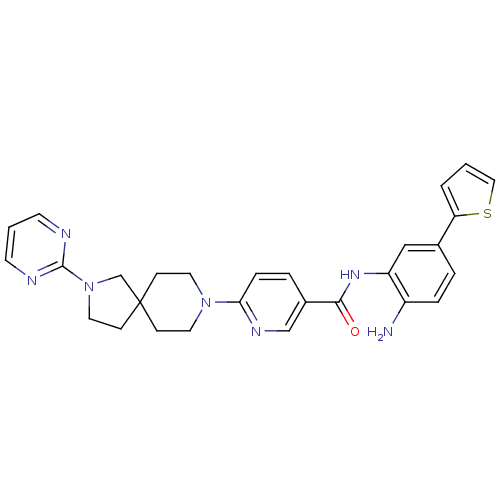
(CHEMBL470798 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCN(C2)c2ncccn2)CC1)-c1cccs1 Show InChI InChI=1S/C28H29N7OS/c29-22-6-4-20(24-3-1-16-37-24)17-23(22)33-26(36)21-5-7-25(32-18-21)34-13-8-28(9-14-34)10-15-35(19-28)27-30-11-2-12-31-27/h1-7,11-12,16-18H,8-10,13-15,19,29H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275770

(CHEMBL513568 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1cccs1 Show InChI InChI=1S/C24H27N5OS/c25-19-5-3-17(21-2-1-13-31-21)14-20(19)28-23(30)18-4-6-22(27-15-18)29-11-8-24(9-12-29)7-10-26-16-24/h1-6,13-15,26H,7-12,16,25H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275824
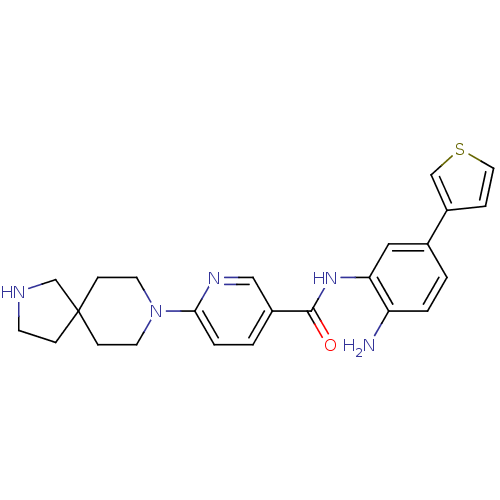
(CHEMBL511797 | N-(2-amino-5-(thiophen-3-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1ccsc1 Show InChI InChI=1S/C24H27N5OS/c25-20-3-1-17(19-5-12-31-15-19)13-21(20)28-23(30)18-2-4-22(27-14-18)29-10-7-24(8-11-29)6-9-26-16-24/h1-5,12-15,26H,6-11,16,25H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275710
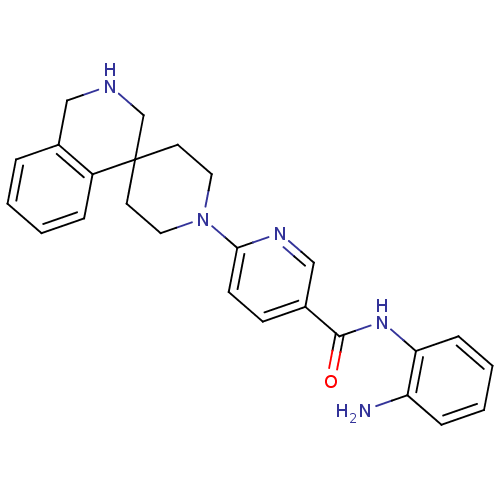
(CHEMBL486738 | N-(2-aminophenyl)-6-(2,3-dihydro-1H...)Show SMILES Nc1ccccc1NC(=O)c1ccc(nc1)N1CCC2(CC1)CNCc1ccccc21 Show InChI InChI=1S/C25H27N5O/c26-21-7-3-4-8-22(21)29-24(31)19-9-10-23(28-16-19)30-13-11-25(12-14-30)17-27-15-18-5-1-2-6-20(18)25/h1-10,16,27H,11-15,17,26H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275940
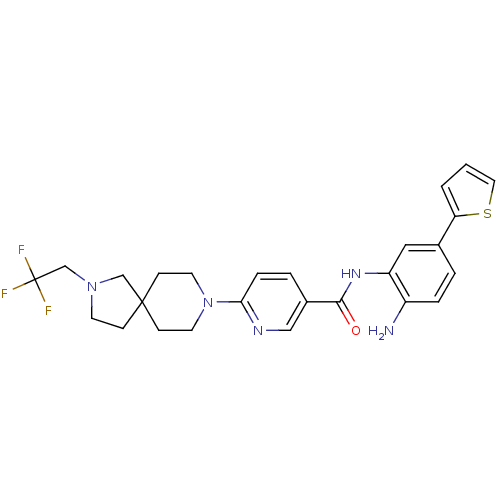
(CHEMBL472261 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCN(CC(F)(F)F)C2)CC1)-c1cccs1 Show InChI InChI=1S/C26H28F3N5OS/c27-26(28,29)17-33-10-7-25(16-33)8-11-34(12-9-25)23-6-4-19(15-31-23)24(35)32-21-14-18(3-5-20(21)30)22-2-1-13-36-22/h1-6,13-15H,7-12,16-17,30H2,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275984
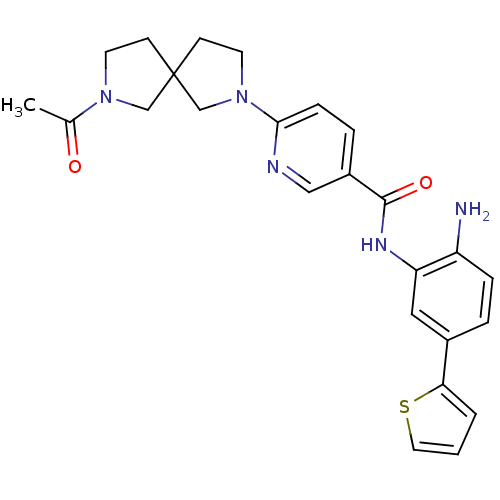
(6-(7-acetyl-2,7-diazaspiro[4.4]nonan-2-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(CCN(C2)c2ccc(cn2)C(=O)Nc2cc(ccc2N)-c2cccs2)C1 Show InChI InChI=1S/C25H27N5O2S/c1-17(31)29-10-8-25(15-29)9-11-30(16-25)23-7-5-19(14-27-23)24(32)28-21-13-18(4-6-20(21)26)22-3-2-12-33-22/h2-7,12-14H,8-11,15-16,26H2,1H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275942
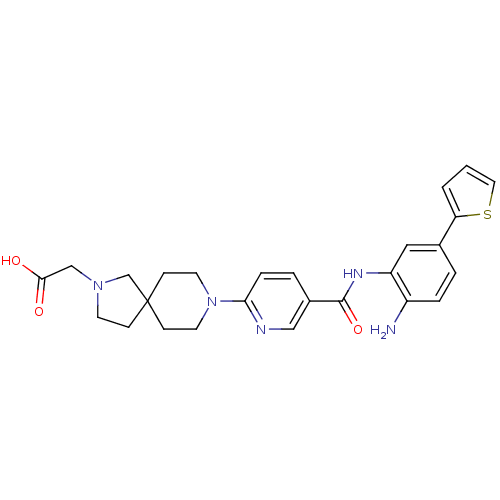
(2-(8-(5-(2-amino-5-(thiophen-2-yl)phenylcarbamoyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCN(CC(O)=O)C2)CC1)-c1cccs1 Show InChI InChI=1S/C26H29N5O3S/c27-20-5-3-18(22-2-1-13-35-22)14-21(20)29-25(34)19-4-6-23(28-15-19)31-11-8-26(9-12-31)7-10-30(17-26)16-24(32)33/h1-6,13-15H,7-12,16-17,27H2,(H,29,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275986
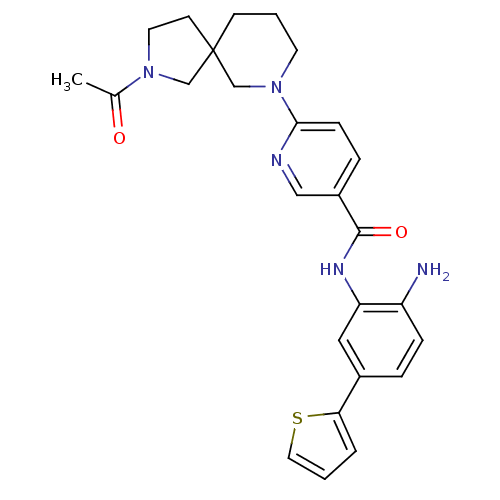
(6-(2-acetyl-2,7-diazaspiro[4.5]decan-7-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(C1)CCCN(C2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H29N5O2S/c1-18(32)30-12-10-26(16-30)9-3-11-31(17-26)24-8-6-20(15-28-24)25(33)29-22-14-19(5-7-21(22)27)23-4-2-13-34-23/h2,4-8,13-15H,3,9-12,16-17,27H2,1H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275887
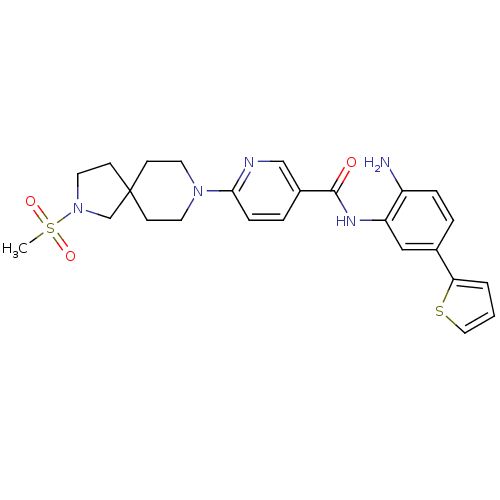
(CHEMBL470582 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES CS(=O)(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C25H29N5O3S2/c1-35(32,33)30-13-10-25(17-30)8-11-29(12-9-25)23-7-5-19(16-27-23)24(31)28-21-15-18(4-6-20(21)26)22-3-2-14-34-22/h2-7,14-16H,8-13,17,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275985

(6-(2-acetyl-2,7-diazaspiro[3.5]nonan-7-yl)-N-(2-am...)Show SMILES CC(=O)N1CC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C25H27N5O2S/c1-17(31)30-15-25(16-30)8-10-29(11-9-25)23-7-5-19(14-27-23)24(32)28-21-13-18(4-6-20(21)26)22-3-2-12-33-22/h2-7,12-14H,8-11,15-16,26H2,1H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275362
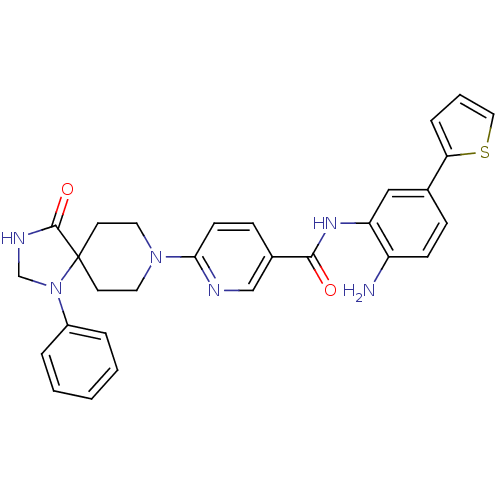
(CHEMBL521193 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CC1)N(CNC2=O)c1ccccc1)-c1cccs1 Show InChI InChI=1S/C29H28N6O2S/c30-23-10-8-20(25-7-4-16-38-25)17-24(23)33-27(36)21-9-11-26(31-18-21)34-14-12-29(13-15-34)28(37)32-19-35(29)22-5-2-1-3-6-22/h1-11,16-18H,12-15,19,30H2,(H,32,37)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276077
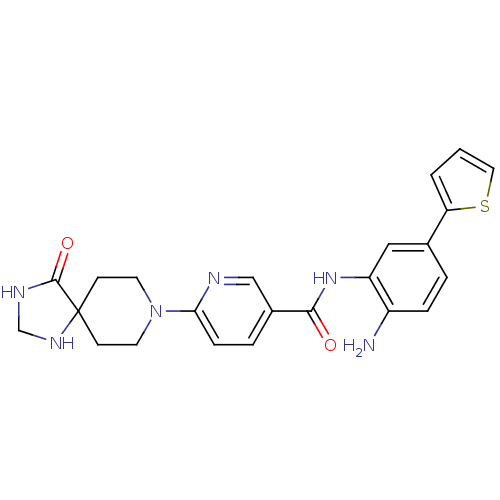
(CHEMBL469950 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CC1)NCNC2=O)-c1cccs1 Show InChI InChI=1S/C23H24N6O2S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)28-21(30)16-4-6-20(25-13-16)29-9-7-23(8-10-29)22(31)26-14-27-23/h1-6,11-13,27H,7-10,14,24H2,(H,26,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276028
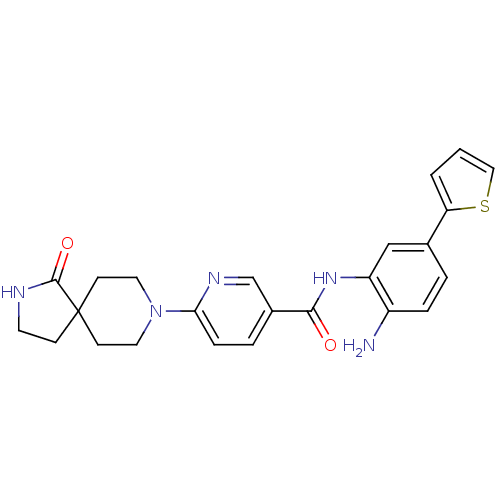
(CHEMBL469514 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2=O)CC1)-c1cccs1 Show InChI InChI=1S/C24H25N5O2S/c25-18-5-3-16(20-2-1-13-32-20)14-19(18)28-22(30)17-4-6-21(27-15-17)29-11-8-24(9-12-29)7-10-26-23(24)31/h1-6,13-15H,7-12,25H2,(H,26,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276075
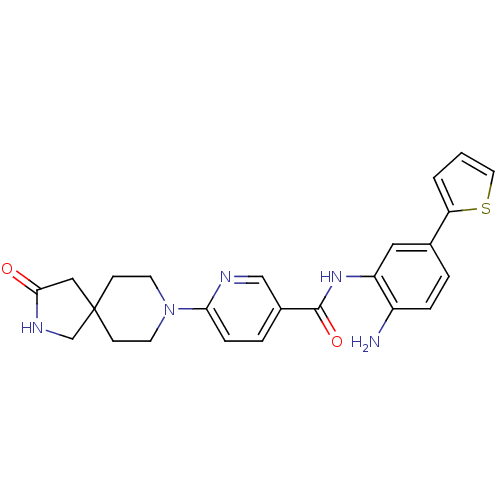
(CHEMBL511984 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC(=O)C2)CC1)-c1cccs1 Show InChI InChI=1S/C24H25N5O2S/c25-18-5-3-16(20-2-1-11-32-20)12-19(18)28-23(31)17-4-6-21(26-14-17)29-9-7-24(8-10-29)13-22(30)27-15-24/h1-6,11-12,14H,7-10,13,15,25H2,(H,27,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276076
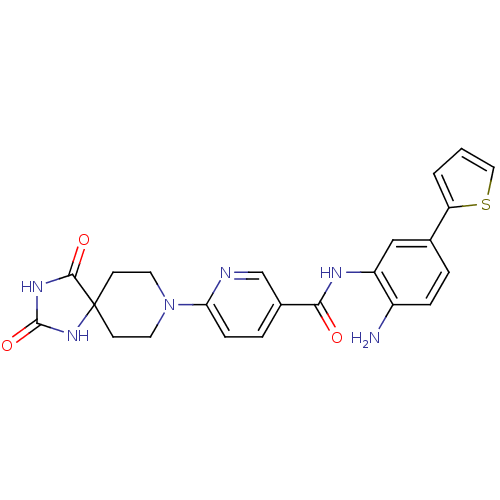
(CHEMBL513027 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CC1)NC(=O)NC2=O)-c1cccs1 Show InChI InChI=1S/C23H22N6O3S/c24-16-5-3-14(18-2-1-11-33-18)12-17(16)26-20(30)15-4-6-19(25-13-15)29-9-7-23(8-10-29)21(31)27-22(32)28-23/h1-6,11-13H,7-10,24H2,(H,26,30)(H2,27,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276027
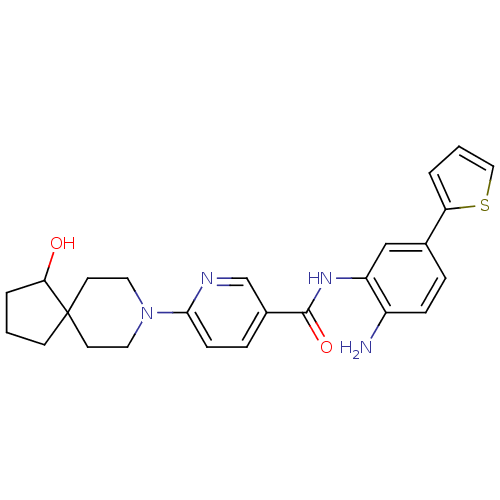
(CHEMBL470148 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCCC2O)CC1)-c1cccs1 Show InChI InChI=1S/C25H28N4O2S/c26-19-7-5-17(21-3-2-14-32-21)15-20(19)28-24(31)18-6-8-23(27-16-18)29-12-10-25(11-13-29)9-1-4-22(25)30/h2-3,5-8,14-16,22,30H,1,4,9-13,26H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275885
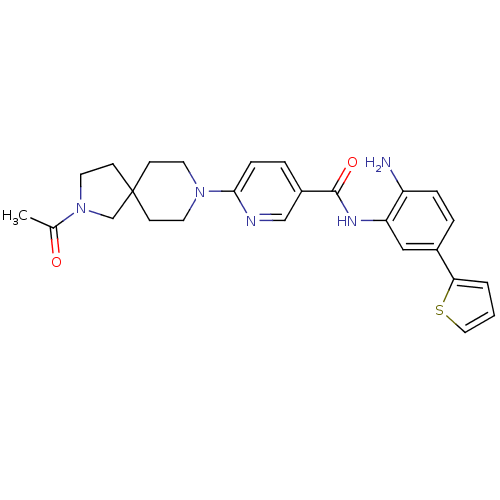
(6-(2-acetyl-2,8-diazaspiro[4.5]decan-8-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H29N5O2S/c1-18(32)31-13-10-26(17-31)8-11-30(12-9-26)24-7-5-20(16-28-24)25(33)29-22-15-19(4-6-21(22)27)23-3-2-14-34-23/h2-7,14-16H,8-13,17,27H2,1H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275827
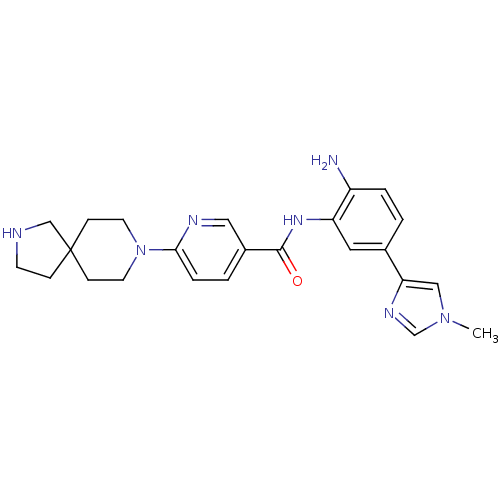
(CHEMBL472252 | N-(2-amino-5-(1-methyl-1H-imidazol-...)Show SMILES Cn1cnc(c1)-c1ccc(N)c(NC(=O)c2ccc(nc2)N2CCC3(CCNC3)CC2)c1 Show InChI InChI=1S/C24H29N7O/c1-30-14-21(28-16-30)17-2-4-19(25)20(12-17)29-23(32)18-3-5-22(27-13-18)31-10-7-24(8-11-31)6-9-26-15-24/h2-5,12-14,16,26H,6-11,15,25H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276026
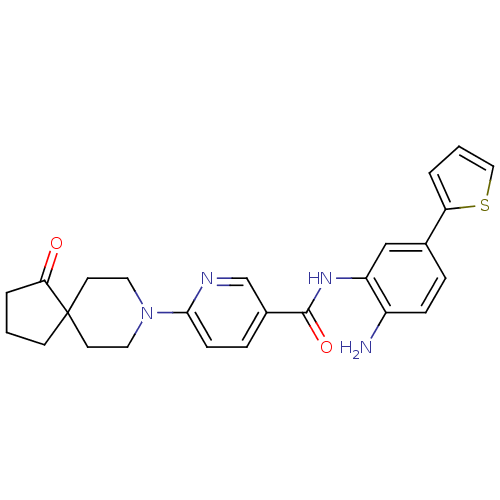
(CHEMBL470147 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCCC2=O)CC1)-c1cccs1 Show InChI InChI=1S/C25H26N4O2S/c26-19-7-5-17(21-3-2-14-32-21)15-20(19)28-24(31)18-6-8-23(27-16-18)29-12-10-25(11-13-29)9-1-4-22(25)30/h2-3,5-8,14-16H,1,4,9-13,26H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275364
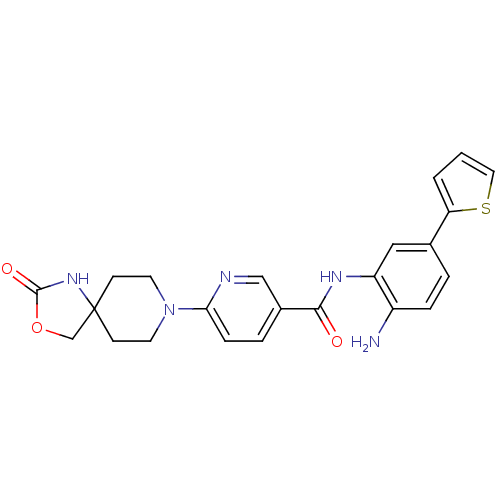
(CHEMBL486770 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(COC(=O)N2)CC1)-c1cccs1 Show InChI InChI=1S/C23H23N5O3S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)26-21(29)16-4-6-20(25-13-16)28-9-7-23(8-10-28)14-31-22(30)27-23/h1-6,11-13H,7-10,14,24H2,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275826
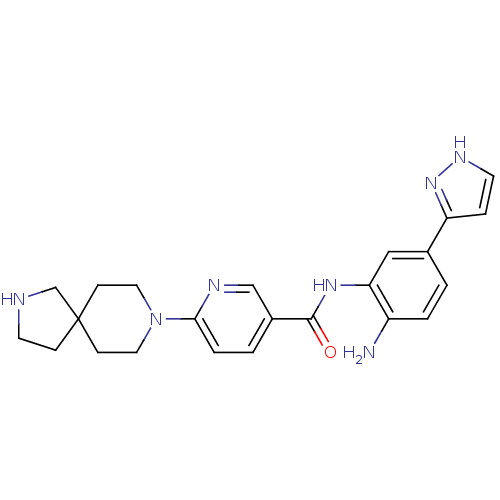
(CHEMBL472251 | N-(2-amino-5-(1H-pyrazol-3-yl)pheny...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1cc[nH]n1 Show InChI InChI=1S/C23H27N7O/c24-18-3-1-16(19-5-9-27-29-19)13-20(18)28-22(31)17-2-4-21(26-14-17)30-11-7-23(8-12-30)6-10-25-15-23/h1-5,9,13-14,25H,6-8,10-12,15,24H2,(H,27,29)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276025
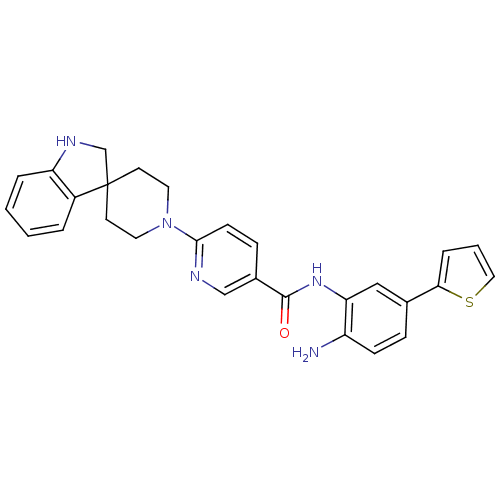
(CHEMBL511625 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNc3ccccc23)CC1)-c1cccs1 Show InChI InChI=1S/C28H27N5OS/c29-22-9-7-19(25-6-3-15-35-25)16-24(22)32-27(34)20-8-10-26(30-17-20)33-13-11-28(12-14-33)18-31-23-5-2-1-4-21(23)28/h1-10,15-17,31H,11-14,18,29H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50276074
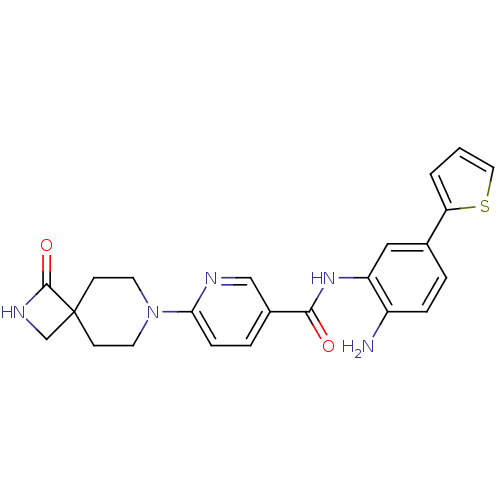
(CHEMBL470791 | HDAC inhibitor, Compound 2 | N-(2-a...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC2=O)CC1)-c1cccs1 Show InChI InChI=1S/C23H23N5O2S/c24-17-5-3-15(19-2-1-11-31-19)12-18(17)27-21(29)16-4-6-20(25-13-16)28-9-7-23(8-10-28)14-26-22(23)30/h1-6,11-13H,7-10,14,24H2,(H,26,30)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275363
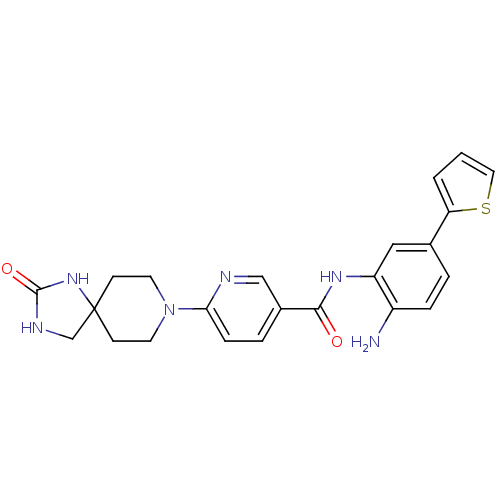
(CHEMBL486152 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC(=O)N2)CC1)-c1cccs1 Show InChI InChI=1S/C23H24N6O2S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)27-21(30)16-4-6-20(25-13-16)29-9-7-23(8-10-29)14-26-22(31)28-23/h1-6,11-13H,7-10,14,24H2,(H,27,30)(H2,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275987
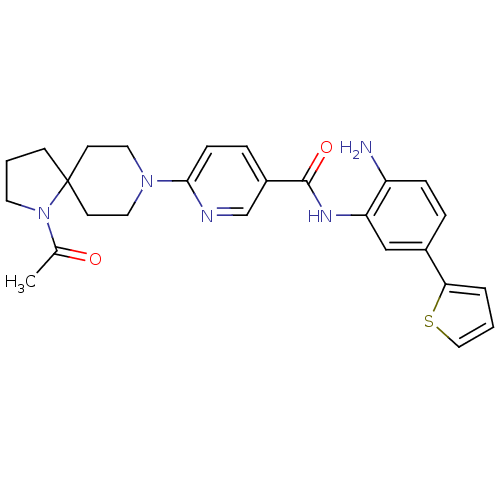
(6-(1-acetyl-1,8-diazaspiro[4.5]decan-8-yl)-N-(2-am...)Show SMILES CC(=O)N1CCCC11CCN(CC1)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H29N5O2S/c1-18(32)31-12-3-9-26(31)10-13-30(14-11-26)24-8-6-20(17-28-24)25(33)29-22-16-19(5-7-21(22)27)23-4-2-15-34-23/h2,4-8,15-17H,3,9-14,27H2,1H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275709
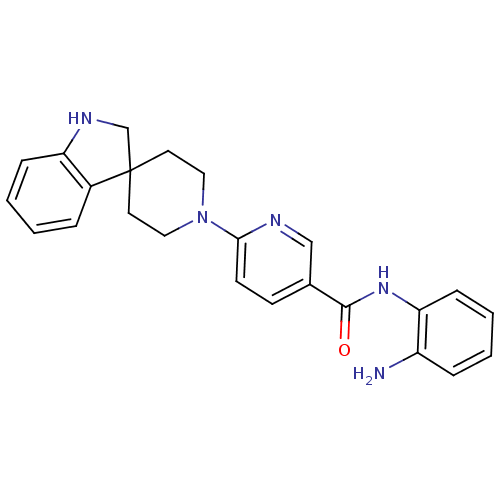
(CHEMBL520050 | N-(2-aminophenyl)-6-(spiro[indoline...)Show SMILES Nc1ccccc1NC(=O)c1ccc(nc1)N1CCC2(CNc3ccccc23)CC1 Show InChI InChI=1S/C24H25N5O/c25-19-6-2-4-8-21(19)28-23(30)17-9-10-22(26-15-17)29-13-11-24(12-14-29)16-27-20-7-3-1-5-18(20)24/h1-10,15,27H,11-14,16,25H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275825
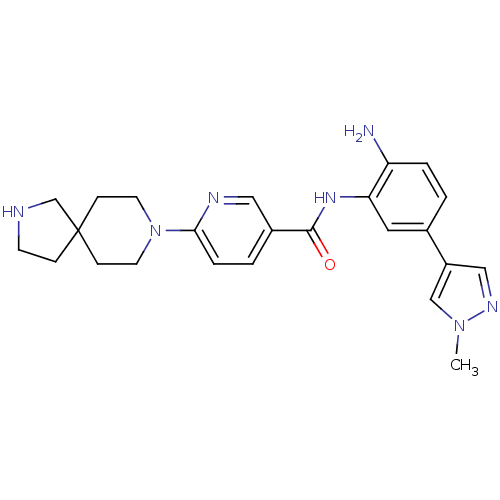
(CHEMBL470156 | N-(2-amino-5-(1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(cn1)-c1ccc(N)c(NC(=O)c2ccc(nc2)N2CCC3(CCNC3)CC2)c1 Show InChI InChI=1S/C24H29N7O/c1-30-15-19(14-28-30)17-2-4-20(25)21(12-17)29-23(32)18-3-5-22(27-13-18)31-10-7-24(8-11-31)6-9-26-16-24/h2-5,12-15,26H,6-11,16,25H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275768
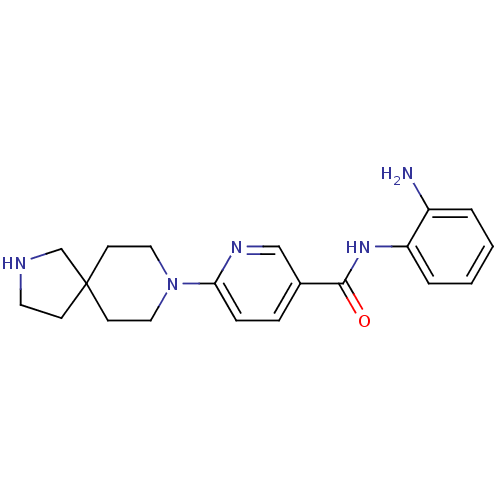
(CHEMBL499776 | N-(2-aminophenyl)-6-(2,8-diazaspiro...)Show InChI InChI=1S/C20H25N5O/c21-16-3-1-2-4-17(16)24-19(26)15-5-6-18(23-13-15)25-11-8-20(9-12-25)7-10-22-14-20/h1-6,13,22H,7-12,14,21H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275708
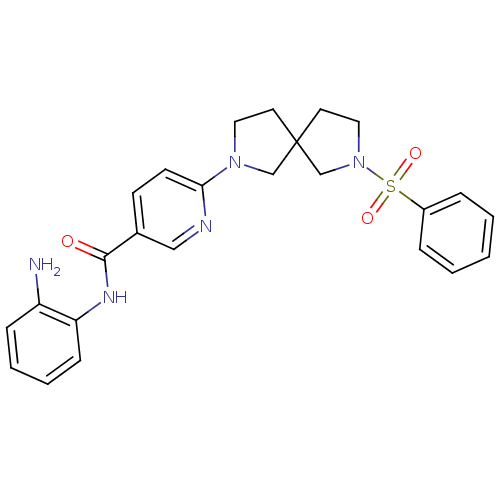
(CHEMBL486737 | N-(2-aminophenyl)-6-(7-(phenylsulfo...)Show SMILES Nc1ccccc1NC(=O)c1ccc(nc1)N1CCC2(CCN(C2)S(=O)(=O)c2ccccc2)C1 Show InChI InChI=1S/C25H27N5O3S/c26-21-8-4-5-9-22(21)28-24(31)19-10-11-23(27-16-19)29-14-12-25(17-29)13-15-30(18-25)34(32,33)20-6-2-1-3-7-20/h1-11,16H,12-15,17-18,26H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275674
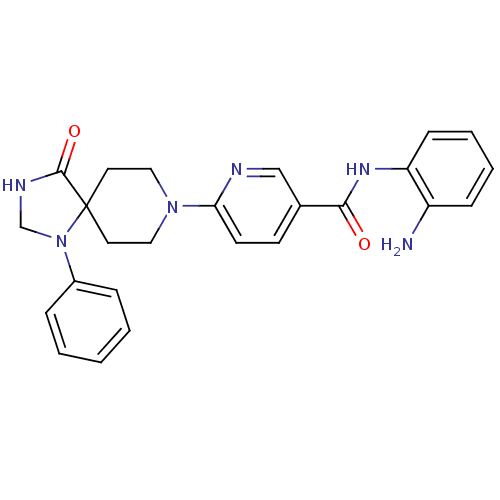
(CHEMBL487346 | N-(2-aminophenyl)-6-(4-oxo-1-phenyl...)Show SMILES Nc1ccccc1NC(=O)c1ccc(nc1)N1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C25H26N6O2/c26-20-8-4-5-9-21(20)29-23(32)18-10-11-22(27-16-18)30-14-12-25(13-15-30)24(33)28-17-31(25)19-6-2-1-3-7-19/h1-11,16H,12-15,17,26H2,(H,28,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275941

(CHEMBL513250 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCN(CCO)C2)CC1)-c1cccs1 Show InChI InChI=1S/C26H31N5O2S/c27-21-5-3-19(23-2-1-15-34-23)16-22(21)29-25(33)20-4-6-24(28-17-20)31-11-8-26(9-12-31)7-10-30(18-26)13-14-32/h1-6,15-17,32H,7-14,18,27H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275363

(CHEMBL486152 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC(=O)N2)CC1)-c1cccs1 Show InChI InChI=1S/C23H24N6O2S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)27-21(30)16-4-6-20(25-13-16)29-9-7-23(8-10-29)14-26-22(31)28-23/h1-6,11-13H,7-10,14,24H2,(H,27,30)(H2,26,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275364

(CHEMBL486770 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(COC(=O)N2)CC1)-c1cccs1 Show InChI InChI=1S/C23H23N5O3S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)26-21(29)16-4-6-20(25-13-16)28-9-7-23(8-10-28)14-31-22(30)27-23/h1-6,11-13H,7-10,14,24H2,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275887

(CHEMBL470582 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES CS(=O)(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C25H29N5O3S2/c1-35(32,33)30-13-10-25(17-30)8-11-29(12-9-25)23-7-5-19(16-27-23)24(31)28-21-15-18(4-6-20(21)26)22-3-2-14-34-22/h2-7,14-16H,8-13,17,26H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275770

(CHEMBL513568 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1cccs1 Show InChI InChI=1S/C24H27N5OS/c25-19-5-3-17(21-2-1-13-31-21)14-20(19)28-23(30)18-4-6-22(27-15-18)29-11-8-24(9-12-29)7-10-26-16-24/h1-6,13-15,26H,7-12,16,25H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275986

(6-(2-acetyl-2,7-diazaspiro[4.5]decan-7-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(C1)CCCN(C2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H29N5O2S/c1-18(32)30-12-10-26(16-30)9-3-11-31(17-26)24-8-6-20(15-28-24)25(33)29-22-14-19(5-7-21(22)27)23-4-2-13-34-23/h2,4-8,13-15H,3,9-12,16-17,27H2,1H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275885

(6-(2-acetyl-2,8-diazaspiro[4.5]decan-8-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H29N5O2S/c1-18(32)31-13-10-26(17-31)8-11-30(12-9-26)24-7-5-20(16-28-24)25(33)29-22-15-19(4-6-21(22)27)23-3-2-14-34-23/h2-7,14-16H,8-13,17,27H2,1H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275824

(CHEMBL511797 | N-(2-amino-5-(thiophen-3-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1ccsc1 Show InChI InChI=1S/C24H27N5OS/c25-20-3-1-17(19-5-12-31-15-19)13-21(20)28-23(30)18-2-4-22(27-14-18)29-10-7-24(8-11-29)6-9-26-16-24/h1-5,12-15,26H,6-11,16,25H2,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275769

(CHEMBL471824 | N-(4-aminobiphenyl-3-yl)-6-(2,8-dia...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCNC2)CC1)-c1ccccc1 Show InChI InChI=1S/C26H29N5O/c27-22-8-6-20(19-4-2-1-3-5-19)16-23(22)30-25(32)21-7-9-24(29-17-21)31-14-11-26(12-15-31)10-13-28-18-26/h1-9,16-17,28H,10-15,18,27H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50276074

(CHEMBL470791 | HDAC inhibitor, Compound 2 | N-(2-a...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC2=O)CC1)-c1cccs1 Show InChI InChI=1S/C23H23N5O2S/c24-17-5-3-15(19-2-1-11-31-19)12-18(17)27-21(29)16-4-6-20(25-13-16)28-9-7-23(8-10-28)14-26-22(23)30/h1-6,11-13H,7-10,14,24H2,(H,26,30)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50276077

(CHEMBL469950 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CC1)NCNC2=O)-c1cccs1 Show InChI InChI=1S/C23H24N6O2S/c24-17-5-3-15(19-2-1-11-32-19)12-18(17)28-21(30)16-4-6-20(25-13-16)29-9-7-23(8-10-29)22(31)26-14-27-23/h1-6,11-13,27H,7-10,14,24H2,(H,26,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275984

(6-(7-acetyl-2,7-diazaspiro[4.4]nonan-2-yl)-N-(2-am...)Show SMILES CC(=O)N1CCC2(CCN(C2)c2ccc(cn2)C(=O)Nc2cc(ccc2N)-c2cccs2)C1 Show InChI InChI=1S/C25H27N5O2S/c1-17(31)29-10-8-25(15-29)9-11-30(16-25)23-7-5-19(14-27-23)24(32)28-21-13-18(4-6-20(21)26)22-3-2-12-33-22/h2-7,12-14H,8-11,15-16,26H2,1H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50276075

(CHEMBL511984 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CNC(=O)C2)CC1)-c1cccs1 Show InChI InChI=1S/C24H25N5O2S/c25-18-5-3-16(20-2-1-11-32-20)12-19(18)28-23(31)17-4-6-21(26-14-17)29-9-7-24(8-10-29)13-22(30)27-15-24/h1-6,11-12,14H,7-10,13,15,25H2,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50276026

(CHEMBL470147 | N-(2-amino-5-(thiophen-2-yl)phenyl)...)Show SMILES Nc1ccc(cc1NC(=O)c1ccc(nc1)N1CCC2(CCCC2=O)CC1)-c1cccs1 Show InChI InChI=1S/C25H26N4O2S/c26-19-7-5-17(21-3-2-14-32-21)15-20(19)28-24(31)18-6-8-23(27-16-18)29-12-10-25(11-13-29)9-1-4-22(25)30/h2-3,5-8,14-16H,1,4,9-13,26H2,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275943

(6-(2-(2-amino-2-oxoethyl)-2,8-diazaspiro[4.5]decan...)Show SMILES NC(=O)CN1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C26H30N6O2S/c27-20-5-3-18(22-2-1-13-35-22)14-21(20)30-25(34)19-4-6-24(29-15-19)32-11-8-26(9-12-32)7-10-31(17-26)16-23(28)33/h1-6,13-15H,7-12,16-17,27H2,(H2,28,33)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50275886

(8-(5-(2-amino-5-(thiophen-2-yl)phenylcarbamoyl)pyr...)Show SMILES CCNC(=O)N1CCC2(C1)CCN(CC2)c1ccc(cn1)C(=O)Nc1cc(ccc1N)-c1cccs1 Show InChI InChI=1S/C27H32N6O2S/c1-2-29-26(35)33-14-11-27(18-33)9-12-32(13-10-27)24-8-6-20(17-30-24)25(34)31-22-16-19(5-7-21(22)28)23-4-3-15-36-23/h3-8,15-17H,2,9-14,18,28H2,1H3,(H,29,35)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in mammalian cells |
Bioorg Med Chem Lett 18: 6104-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.052
BindingDB Entry DOI: 10.7270/Q23R0SQ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data