

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277113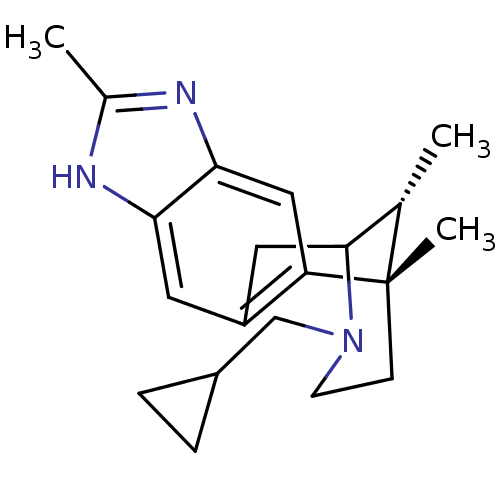 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277101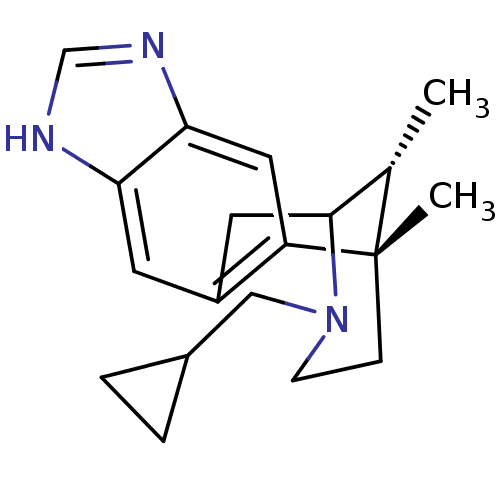 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277118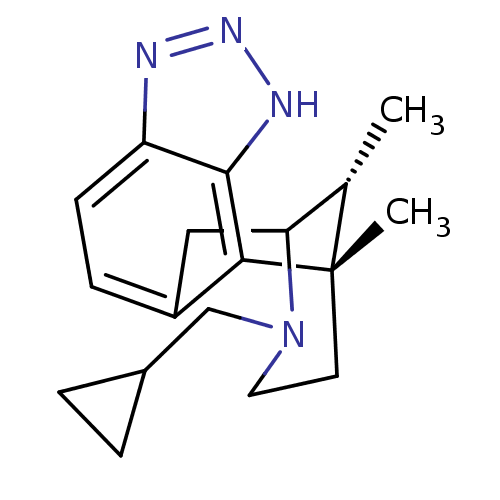 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277101 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277117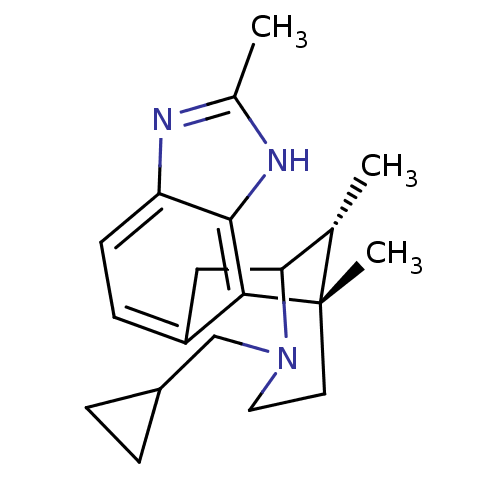 ((1S,16R)-13-(cyclopropylmethyl)-1,5,16-trimethyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277113 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277115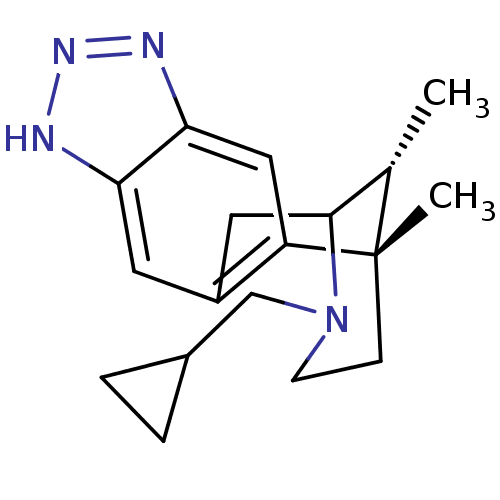 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277116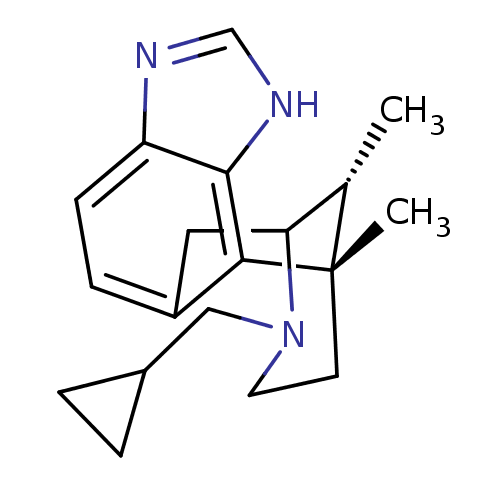 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277118 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277117 ((1S,16R)-13-(cyclopropylmethyl)-1,5,16-trimethyl-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277101 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50124004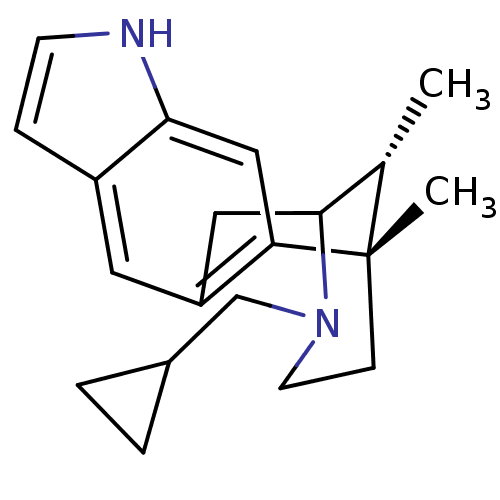 (13-cyclopropylmethyl-1,16-dimethyl-(1S,16R)-5,13-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277116 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277115 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277114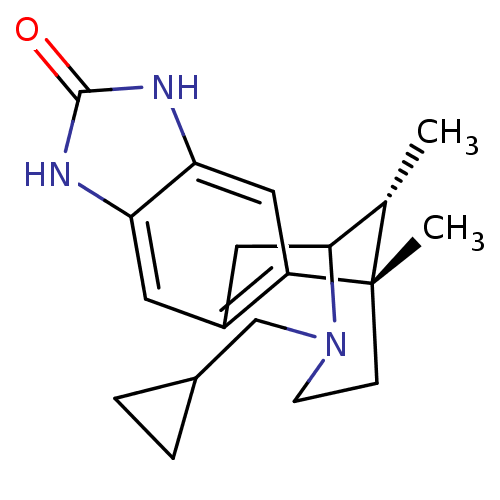 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277113 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50124004 (13-cyclopropylmethyl-1,16-dimethyl-(1S,16R)-5,13-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277118 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277115 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277114 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277117 ((1S,16R)-13-(cyclopropylmethyl)-1,5,16-trimethyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50124004 (13-cyclopropylmethyl-1,16-dimethyl-(1S,16R)-5,13-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277116 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50277114 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277118 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277101 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277113 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277115 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277113 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50277101 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277101 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277113 ((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50277118 ((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 365-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.076 BindingDB Entry DOI: 10.7270/Q2M0458C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||